MERS Vaccine Candidate Effective on Mice

An innovative vaccine candidate that protects against the Middle East Respiratory Syndrome (MERS) was developed by a collaborative team from The University of Texas Medical Branch at Galveston (UTMB), Saudi Arabia and Canada.
This is important news since there is no vaccine currently available to prevent MERS infection.
Nor are there specific antiviral treatments recommended for MERS infections, says the US Centers for Disease Control and Prevention (CDC).
This research team incorporated CD40L into rAd5-based Middle East Respiratory Syndrome Coronavirus (MERS-CoV) S1 vaccine to enhance immunogenicity and efficacy, but also prevent inadvertent pulmonary pathology post-viral challenge.
In this pre-human study, the research team made 2 versions of a potential vaccine and evaluated their effectiveness and safety in mice that were genetically altered to have more human-like immune responses.
After the mice were vaccinated and then infected with MERS-CoV, both vaccines protected the mice against clinical signs of disease and death.
However, one of the vaccines was unable to stop the virus from causing lung damage.
The other potential vaccine, which more selectively targeted cells with CD40, did not result in any lung damage.
“In the past, we’ve mainly focused on developing universal influenza vaccines by targeting the viral proteins to specific cells that have a molecule called CD40 on their surfaces,” said senior author Chien-Te K Tseng, professor in UTMB’s Centers for Biodefense and Emerging Diseases, in a press release.
The MERS virus was first identified in 2012, and can suddenly cause severe and fatal respiratory symptoms, systemic infection and multi-organ failure. In addition to humans, MERS-CoV has been found in camels in several countries.
The CDC says ‘they don’t know for certain where the virus came from. However, it likely came from an animal source.’
Moreover, MERS can be spread from camel-to-human or person-to-person.
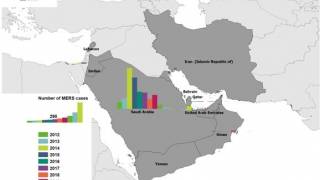
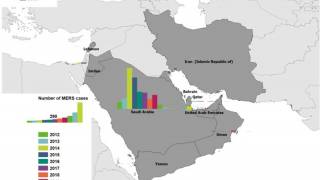
As of February 2019, a total of 2,374 laboratory-confirmed cases of MERS, including 823 associated deaths (case–fatality rate: 34.6%) were reported globally, stated the World Health Organization (WHO) on March 29, 2019.
The majority of these cases were reported from Saudi Arabia, 1983 cases, including 745 related deaths, with a case–fatality rate of 37.5%.
During the month of February 2019, a total of 76 laboratory-confirmed cases of MERS were reported globally: 68 of the cases were reported from Saudi Arabia with 10 associated deaths and 8 were reported from Oman with 2 associated deaths.
Only two patients in the USA have tested positive for MERS-CoV infection, both in May 2014 after recently traveled from Saudi Arabia, says the CDC.
These findings recently were published in The Journal of Infectious Diseases.
Other study authors include UTMB’s Abdullah Algaissi, Anurodh Agrawal and Bi-Hung Peng, as well as Sawsan Al-amri, Rowa Alhabbab and Sayed Sohrab from King Abdulaziz University in Saudi Arabia; Abdulrahman Almasoud and Naif Khalaf Alharbi from King Abdullah International Medical Research Center in Saudi Arabia and Marsha Russell and Xuguang Li from Health Canada.
No conflicts of interest were disclosed.
Our Trust Standards: Medical Advisory Committee
- A highly immunogenic, protective and safe adenovirus-based vaccine expressing MERS-CoV S1-CD40L fusion protein in transgenic
- Researchers develop new vaccine against deadly Middle East Respiratory Syndrome
- Middle East respiratory syndrome coronavirus (MERS-CoV)
- Mers Situation Update: February 2019
- Middle East Respiratory Syndrome (MERS) Prevention and Treatment