5th MERS Cases Confirmed in the Kingdom of Saudi Arabia

The Ministry of Health of the Kingdom of Saudi Arabia (KSA) notified the World Health Organization (WHO) today of one human case of Middle East respiratory syndrome coronavirus (MERS-CoV).
The patient was discharged on September 13, 2024, after receiving a negative test result for this viral respiratory infection. This follow-up appointment was completed, confirming the patient's full recovery.
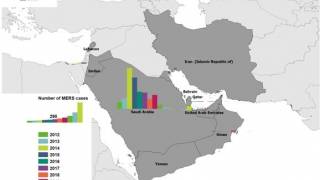
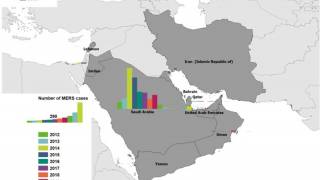
Published on October 2, 2024, this WHO Disease Outbreak News article confirmed that five MERS-CoV cases, including four deaths, have been reported from KSA this year.
Since the first report of MERS-CoV in the KSA in 2012, human infections have been reported in 27 countries, spanning all six WHO regions. Most (84%) MERS-CoV cases (2,205) and related fatalities (960) have been reported in KSA.
The WHO expects that additional cases of MERS-CoV infection will be reported from the Middle East and/or other countries where MERS-CoV is circulating in dromedaries (camels). In addition, cases will continue to be exported to other countries by infected individuals.
People acquire MERS-CoV through direct or indirect interaction with dromedary camels, which serve as the virus's natural host and zoonotic reservoir.
To date, instances of non-sustained human-to-human transmission have been observed primarily among close contacts and within healthcare environments. There has been limited human-to-human transmission outside of healthcare settings.
However, the notification of this new case last month does not change WHO's overall MERS-CoV risk assessment, which remains moderate at both the global and regional levels.
WHO does not advise special screening at points of entry regarding this event, nor does it currently recommend the application of any travel or trade restrictions.
At present, no MERS vaccine or targeted treatment is available.
The WHO says several vaccine candidates are conducting human clinical trials in 2024. These vaccines are based on DNA platforms, viral vector platforms, and modified vaccinia Ankara.
The U.S. Food and Drug Administration and the KSA have not approved a MERS vaccine candidate.
Our Trust Standards: Medical Advisory Committee