Preparing Lyme Disease Defense Starts Now
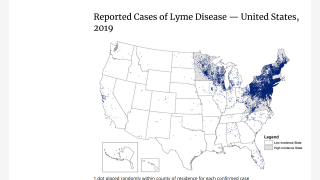
Without an approved Lyme disease vaccine, a bacterial tickborne disease continues to spread in Europe and the United States. Lyme disease cases were initially detected in Lyme, Connecticut, and are now the most common vectorborne disease.
Lyme disease cases have increased by over 100% during the past few years.
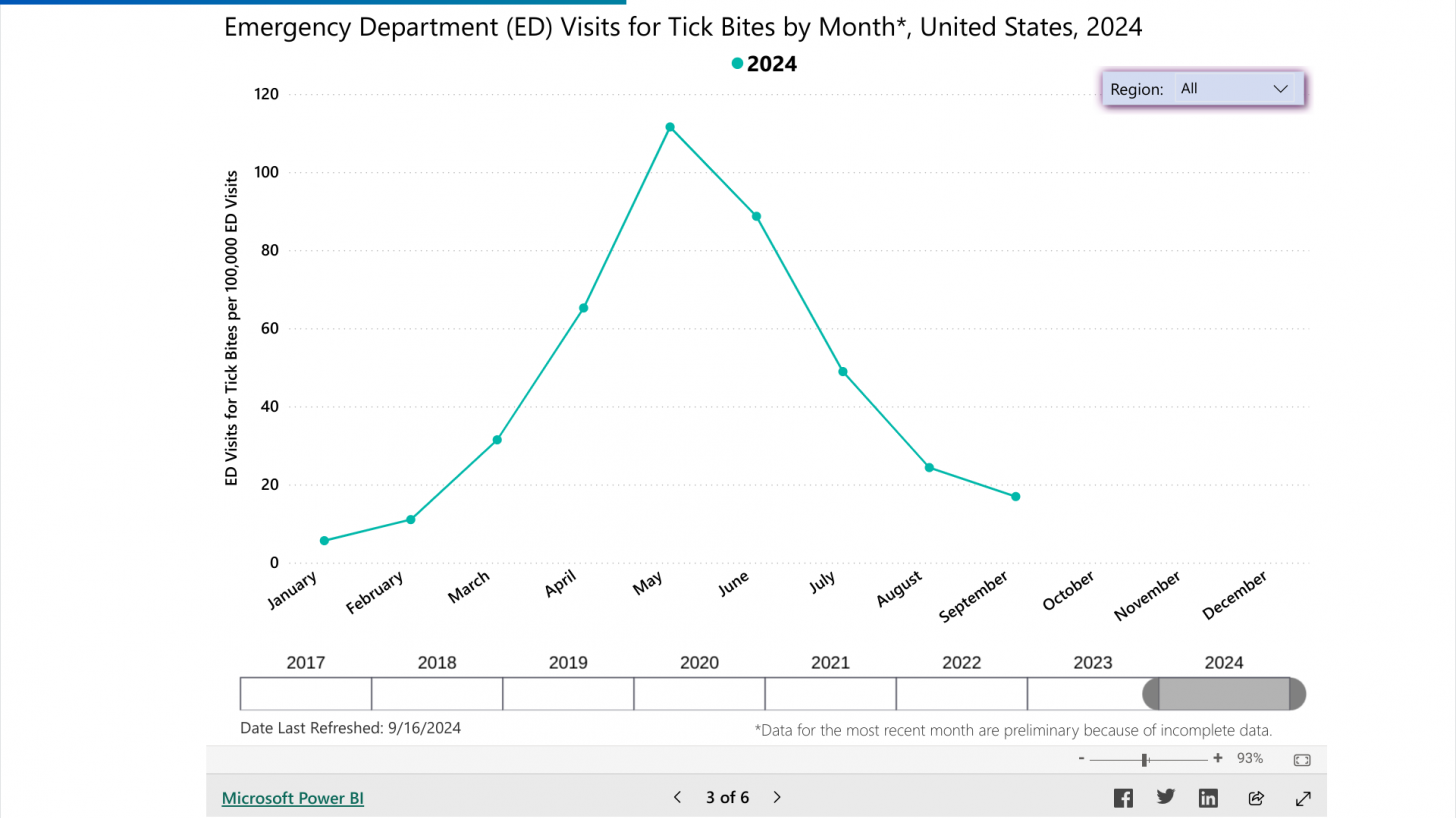
According to the U.S. Centers for Disease Control and Prevention (CDC) Tick Bite dashboard on September 22, 2024, most people are infected in the northeast and midwest areas of the U.S., and peaking around the month of May each year.
Specifically, Lyme disease is most often detected in young and older people.
While most Lyme disease patients recover after treatment, about 5 to 10% can have prolonged symptoms, such as fatigue, pain, and difficulty thinking, as a result of their infection.
Some patients are concerned that their long-term symptoms may be due to Lyme disease and may take potentially harmful treatments, such as extended courses of antibiotics, says the CDC.
In partnership with the CDC, the American Medical Association developed a new information toolkit to assist clinicians in providing better patient care.
During a COCA Call on September 19, 2024, presenters Erica Kaufman West, MD, FACP, FIDSA, and Grace Marx, MD, MPH, FIDSA, shared an overview of Lyme disease, provided a diagnostic and management framework for patients with prolonged symptoms and concerns about Lyme disease, and reviewed new clinical tools and resources to help support these patients.
From a prevention perspective, there are no Lyme disease-approved vaccines as of late September 2024.
However, Valneva SE's VLA15 vaccine candidate has advanced the furthest along the clinical development timeline, with two Phase 3 trials in progress.
The latest results from the VLA15-221 Phase 2 study demonstrated a significant anamnestic antibody response across all six serotypes covered by the vaccine candidate in pediatric and adolescent participants, as well as in adults, measured one month after administration of this second booster dose (month 31).
A high proportion of participants seroconverted after the second booster dose, yielding seroconversion rates (SCRs) above 90% for all outer surface protein A (OspA) serotypes in all age groups, in line with SCRs after the first booster.
Geometric Mean Titers were comparably high one month after the first and second booster (i.e., month 19 vs. month 31).
On September 3, 2024, Juan Carlos Jaramillo, M.D., Chief Medical Officer of Valneva, commented in a press release, "We are encouraged by these (phase 3) data, which support the potential benefit of booster doses across all examined age groups. Each new set of positive data brings us one step closer to potentially bringing this vaccine to adults and children living in areas where Lyme disease is endemic."
Subject to positive phase 3 study data, Valneva and its co-developer Pfizer Inc. could submit a Biologics License Application to the U.S. FDA as early as 2025 and a Marketing Authorization Application to the European Medicines Agency in 2026,
Our Trust Standards: Medical Advisory Committee