Ticks Are Tiny Lyme Disease Carriers
With nearly 900 species of ticks identified globally, their impact on human health is becoming increasingly concerning. Unfortunately, the northeastern United States has become a prime area for these disease-carrying ticks.
According to a study published by the journal Ticks and Tick-borne Diseases in November 2023, there are 20 tick-borne diseases found in the U.S.
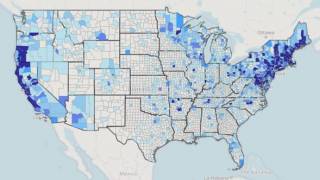
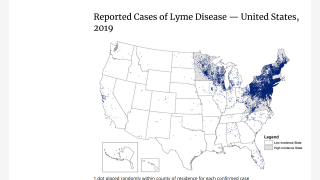
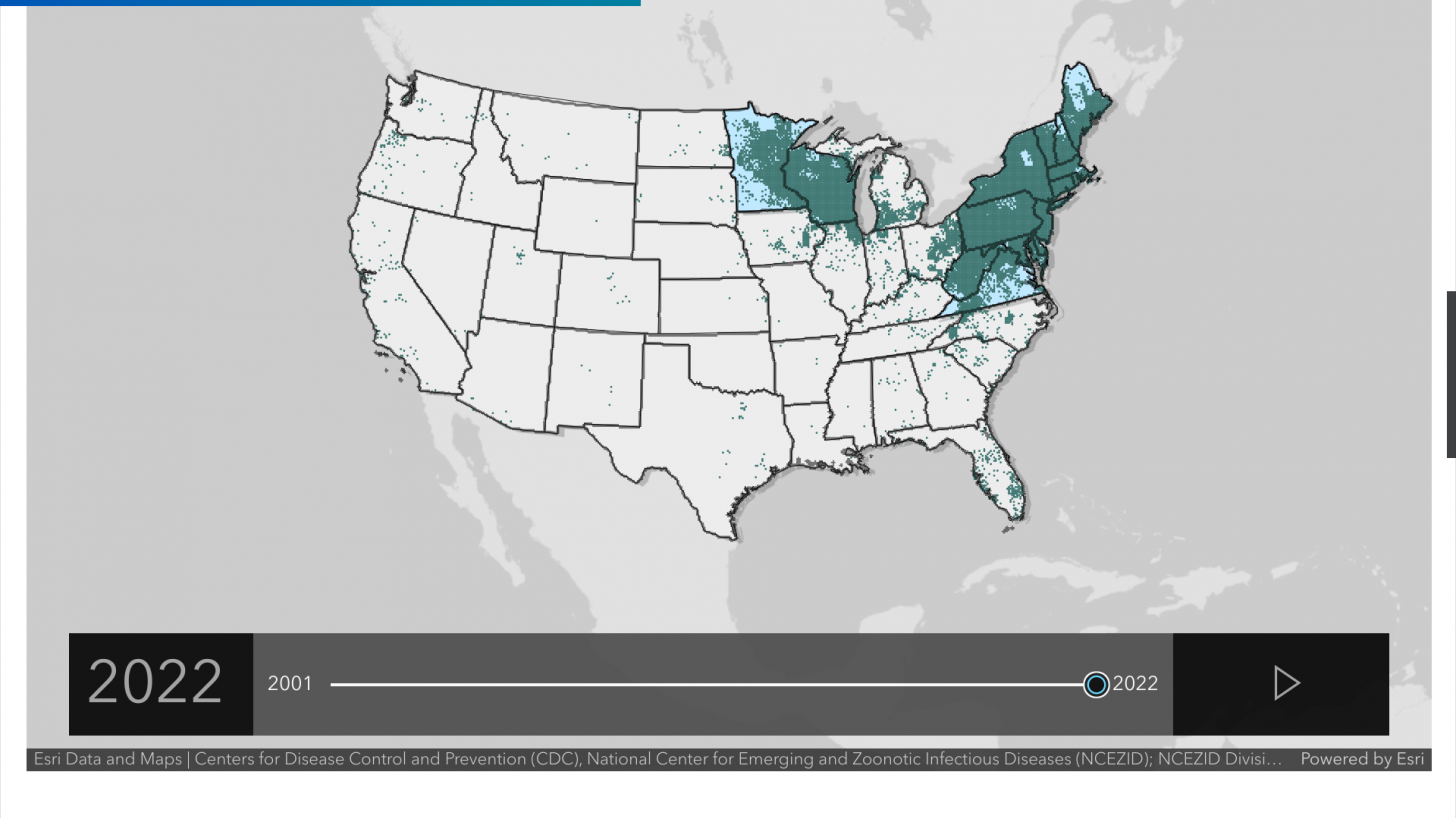
Over time, the black-legged (deer) tick that carries Lyme disease has spread across several states. As its name implies, this disease was first identified in Lyme, Connecticut, and has spread west to states such as Minnesota.
The good news for hikers and campers is that getting bitten by a tick doesn't guarantee you'll become infected with a disease. Most pathogens can only be transmitted if a tick feeds on a host for a particular duration.
In the case of Lyme disease, the risk of illness following a tick bite is 1-5%.
The U.S. Centers for Disease Control and Prevention (CDC) reports that approximately 30,000 to 50,000 cases of Lyme disease are reported in the U.S. each year, with 95% of cases occurring in 15 states.
However, it is estimated that as many as 475,000 people could be infected with Lyme disease annually.
Lyme disease is caused by the Borrelia burgdorferi bacteria, which evades the immune system at the tick bite, leading to flu-like symptoms. Certain strains of the bacteria can cause more severe symptoms, such as neurological and cardiac issues, which occur in about 5% of Lyme cases.
The CDC suggests several ways to prevent tick bites, such as spraying insecticide on clothing and boots, using repellents such as DEET, and checking for ticks after spending time outdoors.
Shower promptly after being in tick habitats—within 2 hours—to help wash off crawling ticks.
Tumble dry clothing on high to kill ticks in clothing. Ticks do not like low humidity levels in homes and will rapidly desiccate and die if they cannot find a host.
According to recent news, a human vaccine for Lyme disease is conducting late-stage phase 3 clinical trials, with expected completion in 2025.
The VLA15 vaccine candidate is a multivalent recombinant protein vaccine targeting Borrelia's outer surface protein A. The U.S. Food and Drug Administration (FDA) granted the VLA15 vaccine development program Fast Track designation.
Recently, Valneva SE and Pfizer, Inc. began collaborating on VLA15's development and commercialization.
Juan Carlos Jaramillo, M.D., Chief Medical Officer, Valneva, said in a press release on July 17, 2024, "We are pleased to see the progress of our Phase 3 VALOR trial. Lyme disease is the most prevalent vector-borne disease in the United States and Europe. It can result in debilitating complications and extensive healthcare treatments."
"Given the growing burden, high medical need, and lack of effectiveness with current interventions, there is an urgent need for novel approaches to help prevent Lyme disease."
Subject to positive data from the VALOR study, Pfizer plans to submit a Biologics License Application to the FDA and a Marketing Authorization Application to the European Medicines Agency in 2026.
As summer vacations end and the fall outdoor season accelerates, the CDC advises everyone to be aware of tiny disease-carrying ticks that may appear where they are least expected.
Our Trust Standards: Medical Advisory Committee