Hexavalent Lyme Disease Vaccine Elicited Antibody Responses to OspA Serotypes

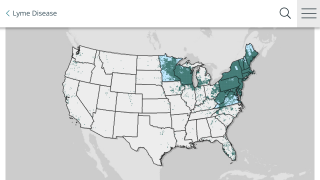
Lyme disease is recognized as the most common vector-borne illness in the Northern Hemisphere, but its actual incidence rate is unknown. It is estimated that over 400,000 people in the U.S. and 129,000 in Europe are diagnosed and treated for Lyme disease each year.
While the disease can be treated, many outdoor enthusiasts eagerly await a preventive vaccine's approval.
In February 2024, results from a survey indicated about 68% of parents in high- and emerging-incidence states would vaccinate their children against Lyme disease.
To address this pent-up consumer demand, the most advanced Lyme disease vaccine candidate announced encouraging news today.
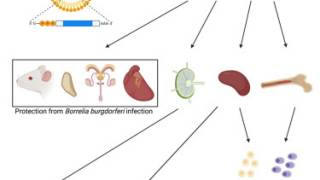
On June 3, 2024, Valneva SE reported that the results of two Phase 2 clinical trials of Lyme disease vaccine candidate VLA15 were published in The Lancet Infectious Diseases. VLA15 is a hexavalent vaccine targeting the most prevalent Borrelia species (serotypes 1-6).
These trials and a third Phase 2 trial in pediatric participants supported the design of the current pivotal Phase 3 trial, VALOR.
The company says VLA15 has advanced the furthest of any Lyme vaccine candidates currently in clinical development.
Juan Carlos Jaramillo, M.D., Chief Medical Officer of Valneva, said in a press release, "We are pleased that these results are now fully available to the broader infectious disease community..... we are excited about the ongoing trials and progress towards potentially offering a vaccine against this unmet medical need."
The article, titled "Optimization of Dose Level and Vaccination Schedule for the VLA15 Lyme Borreliosis Vaccine Candidate Among Healthy Adults: Two Randomized, Phase 2 Studies" provides a detailed analysis of the VLA15-201 and VLA15-202 trial results, which investigated different dose levels and vaccination schedules of VLA15.
VLA15 was immunogenic across all dose groups and vaccination schedules tested.
The most potent antibody responses across all six serotypes were exhibited at the highest dose (180 µg) and broader vaccination intervals (Month 0,2,6). VLA15 has shown a favorable safety and tolerability profile across all trials to date.
Also, an independent Data Safety Monitoring Board observed no safety concerns in any treatment group.
The Phase 3 clinical trial, VALOR, is currently ongoing to investigate the efficacy, safety, and immunogenicity of VLA15 in participants five years of age and older in highly endemic regions in the United States, Canada, and Europe.
Unfortunately, VLA15 may not become available until 2026, when Valneva's development partner Pfizer aims to submit a Biologic License Application to the U.S. Food and Drug Administration and a Marketing Authorization Application to the European Medicines Agency.
The companies previously reported positive results for the booster phase of trial VLA15-202 and a third Phase 2 trial, VLA15-221, providing further evidence of VLA15's safety profile and its potential to provide immunity against Lyme disease in adult, pediatric, and adolescent populations.
Our Trust Standards: Medical Advisory Committee