Phase 2 Clinical Trial Begins for Sudan Ebolavirus Vaccine Candidate

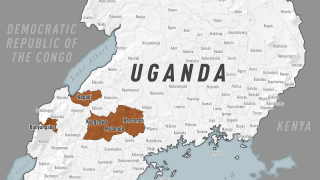
The Sabin Vaccine Institute recently announced the launch of a Phase 2 clinical trial for its vaccine against Sudan ebolavirus at Makerere University Walter Reed Project (MUWRP) in Uganda.
This is a vital development as there are currently no approved vaccines for this strain of ebolavirus.
Based on the cAd3 platform, Sabin’s single-dose investigational Sudan ebolavirus vaccine was found to be promising in Phase 1 clinical and non-clinical studies. Results showed it to be safe while eliciting rapid and robust immune responses that lasted up to 12 months.
“We are delighted to advance a vaccine candidate that can thwart a deadly and devastating disease, especially one that caused a fairly recent outbreak and for which no approved treatments exist,” commented Amy Finan, Sabin’s Chief Executive Officer, in a press release on July 15, 2024.
“Sabin’s vaccine candidate is backed by strong safety and immunogenicity data, and we hope this trial will yield further evidence to move the vaccine closer to licensure.”
This is Sabin’s second Phase 2 clinical trial partnership with MUWRP, based in Uganda’s capital, Kampala. A Phase 2 trial for a Marburg vaccine is already underway, having recently completed enrollment. Initial results from the Marburg trial are expected later this year.
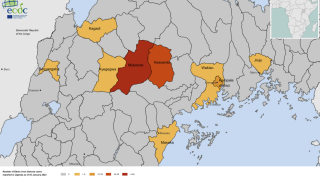
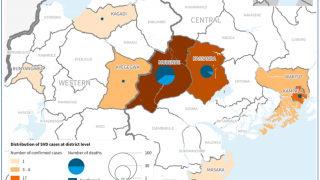
The most recent outbreak of Sudan ebolavirus occurred in Uganda in the fall of 2022. That outbreak ultimately resulted in 55 deaths.
Sabin’s vaccine candidate was the first to arrive in Uganda during that outbreak after the WHO included it as one of three vaccines for possible use in an outbreak trial. The outbreak ended before the vaccine was deployed.
In August 2019, Sabin announced agreements with GSK to advance the development of vaccines against the Zaire and Sudan ebolavirus and Marburg virus. The three candidate vaccines were initially developed collaboratively by the U.S. National Institutes of Health and Okairos, acquired by GSK in 2013.
As of July 20, 2024, the U.S. FDA has approved Zaire Ebolavirus vaccines, which have been offered in Africa since 2019.
Our Trust Standards: Medical Advisory Committee