Norovirus Vaccine Search Continues

HilleVax, Inc., a company focused on developing and commercializing novel vaccines, announced financial results for the June 30, 2024 quarter and highlighted recent progress.
As of June 30, 2024, and December 31, 2023, the company had cash, cash equivalents, and marketable securities totaling $245 million and $303.5 million, respectively.
On August 8, 2024, the company confirmed it is exploring the potential for continued development of its HIL-214 and HIL-216 norovirus vaccine candidates in adults.
However, the company has discontinued further development of HIL-214 in infants.
This is unfortunate news since no U.S. FDA-approved norovirus vaccines are available to meet disease prevention needs.
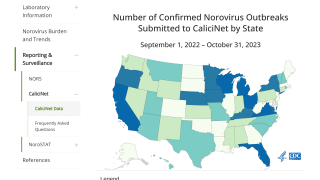
The U.S. CDC recently reported norovirus is the leading cause of vomiting and diarrhea from acute gastroenteritis among people of all ages and causes 58% of foodborne illnesses acquired in the United States.
Each year, there are about 2,500 reported norovirus outbreaks in the U.S., including on cruise ships.
Our Trust Standards: Medical Advisory Committee