More Marburg Vaccines Delivered to Rwanda

In continued collaboration with the Republic of Rwanda, the Sabin Vaccine Institute announced it dispatched approximately 1,000 additional investigational vaccine doses for a randomized clinical trial arm within the ongoing open-label study targeting Marburg virus disease, which causes deadly viral hemorrhagic fever.
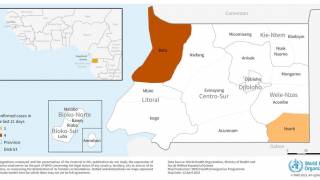
On October 31, 2024, Sabin confirmed that over 1,700 vaccines had already been delivered to Rwanda since September 27. The initial part of the trial focused mainly on health workers, who suffered the most casualties in this outbreak.
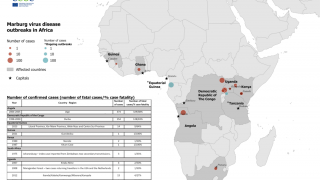
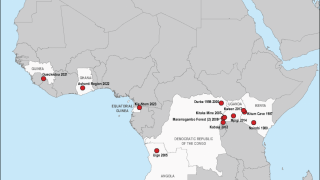
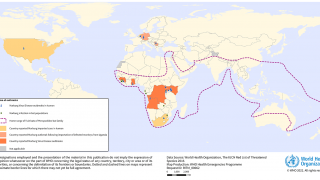
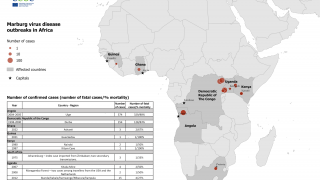
Rwanda has confirmed 66 Marburg cases and 15 related deaths in one of the most significant recorded outbreaks of this disease, which was first detected in Germany in 1967.
Marburg is spread by contact with objects, blood, or body fluids of a person infected with or who has died from Marburg.
Designed to prevent illness before exposure to the virus, Sabin’s Marburg vaccine based on the cAd3 platform has not yet been proven to have clinical benefit for vaccine recipients. The candidate is currently in Phase 2 trials in Uganda and Kenya ; no safety concerns have been reported. In Phase 1 trials, safety and immunogenicity were shown in humans.
Sabin is also a key partner in MARVAC, a WHO-coordinated effort promoting global collaboration in Marburg vaccine development.
As of November 1, 2024, the U.S. CDC says, 'Reconsider nonessential travel to the Republic of Rwanda, which is experiencing an outbreak of Marburg virus disease.'
Our Trust Standards: Medical Advisory Committee