5th COVID-19 Vaccine Launches Phase 3 Study
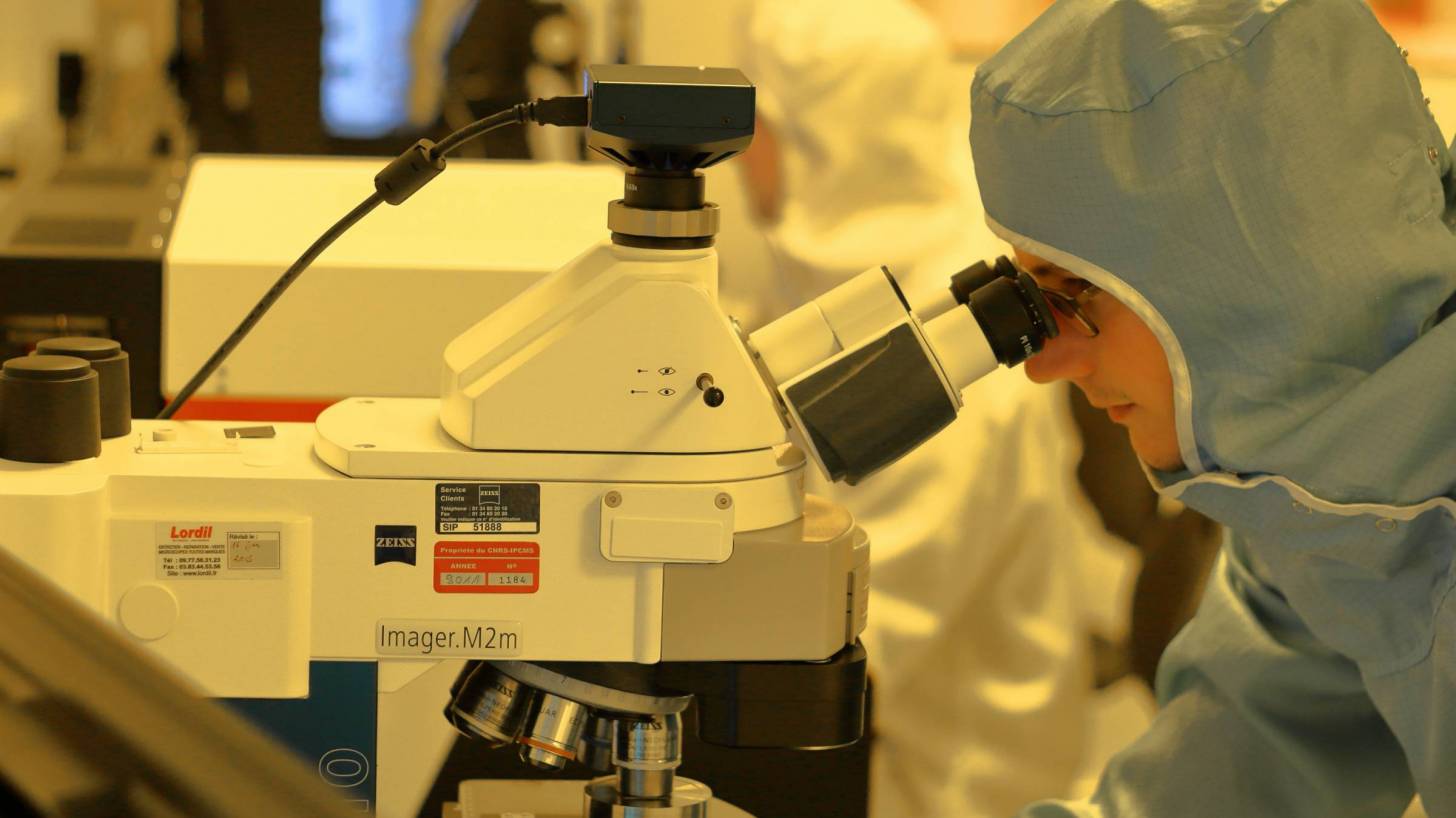
A Maryland-based biotechnology company announced the initiation of PREVENT-19, its pivotal Phase 3 study in the United States and Mexico to evaluate the efficacy, safety, and immunogenicity of NVX-CoV2373, the Company’s COVID-19 vaccine candidate.
Novavax, Inc. stated on December 28, 2020; the Phase 3 trial has begun enrolling adult volunteers into the randomized, placebo-controlled trial, which intends to enroll approximately 30,000 people at about 115 sites.
The trial’s primary endpoint is to determine whether NVX-CoV2373 can prevent symptomatic COVID-19 disease seven or more days after the second injection relative to placebo.
The US National Institute of Allergy and Infectious Diseases (NIAID) and the Biomedical Advanced Research and Development Authority provided funding for the trial.
“Addressing the unprecedented health crisis of COVID-19 has required extraordinary efforts on the part of government, academia, industry and the community,” said NIAID Director Anthony S. Fauci, M.D., in a press release.
“The launch of this study, the fifth investigational COVID-19 vaccine candidate to be tested in a Phase 3 trial in the USA, demonstrates our resolve to end the pandemic through the development of multiple safe and effective vaccines.”
Volunteers will be asked to give informed consent before they participate in the trial. They will be grouped into two cohorts: individuals 18 through 64 years old and those aged 65 and older, to enroll at least 25% of all volunteers who are 65 years old or older.
The trial organizers also are emphasizing recruitment of people who are at higher risk of severe COVID-19 disease, including those who are Black (including African Americans), Native American, or of Latino or Hispanic ethnicity, and people who have underlying health conditions such as obesity, chronic kidney disease or diabetes.
“We’ve come this far, this fast, but we need to get to the finish line,” added NIH Director Francis S. Collins, M.D., Ph.D. “That will require multiple vaccines using different approaches to ensure everyone is protected safely and effectively from this deadly disease.”
After providing a baseline nasopharyngeal and blood sample, participants will be randomly assigned to receive an intramuscular injection of either the investigational vaccine or a saline placebo. Randomization will be in a 2:1 ratio, with two volunteers receiving the investigational vaccine for each one who gets a placebo. Because the trial is blinded, neither investigators nor participants will know who is receiving the candidate vaccine.
A second injection will be administered 21 days after the first.
Of note, specialized assays will be used to distinguish between immunity due to natural infection and vaccine-induced immunity.
Novavax’s investigational vaccine, NVX-CoV2373, is made from a stabilized form of the coronavirus spike protein using its recombinant protein nanoparticle technology. The purified protein antigens in the vaccine cannot replicate and cannot cause COVID-19. The vaccine also contains a proprietary adjuvant, MatrixM™.
Adjuvants are additives that enhance desired immune system responses to a vaccine. NVX-CoV2373 is administered in liquid form and can be stored, handled, and distributed at above-freezing temperatures (35° to 46°F). A single vaccine dose contains five micrograms (mcg) of protein and 50 mcg of adjuvant.
Novavax also recently completed enrollment of more than 15,000 volunteers in a Phase 3 trial of the candidate vaccine in the United Kingdom, testing two injections of 5 mcg of protein and 50 mcg of Matrix-M adjuvant administered 21 days apart.
Adults who are interested in joining this study can visit ClinicalTrials.gov and search identifier NCT04611802.
The National Institutes of Health is the nation's medical research agency, includes 27 Institutes and Centers, and is a component of the U.S. Department of Health and Human Services. For more information about NIH and its programs, visit www.nih.gov.
PrecisionVaccinations publishes research-based vaccine development new.
Our Trust Standards: Medical Advisory Committee