Breast Cancer Remains Most Clinically Studied Disease
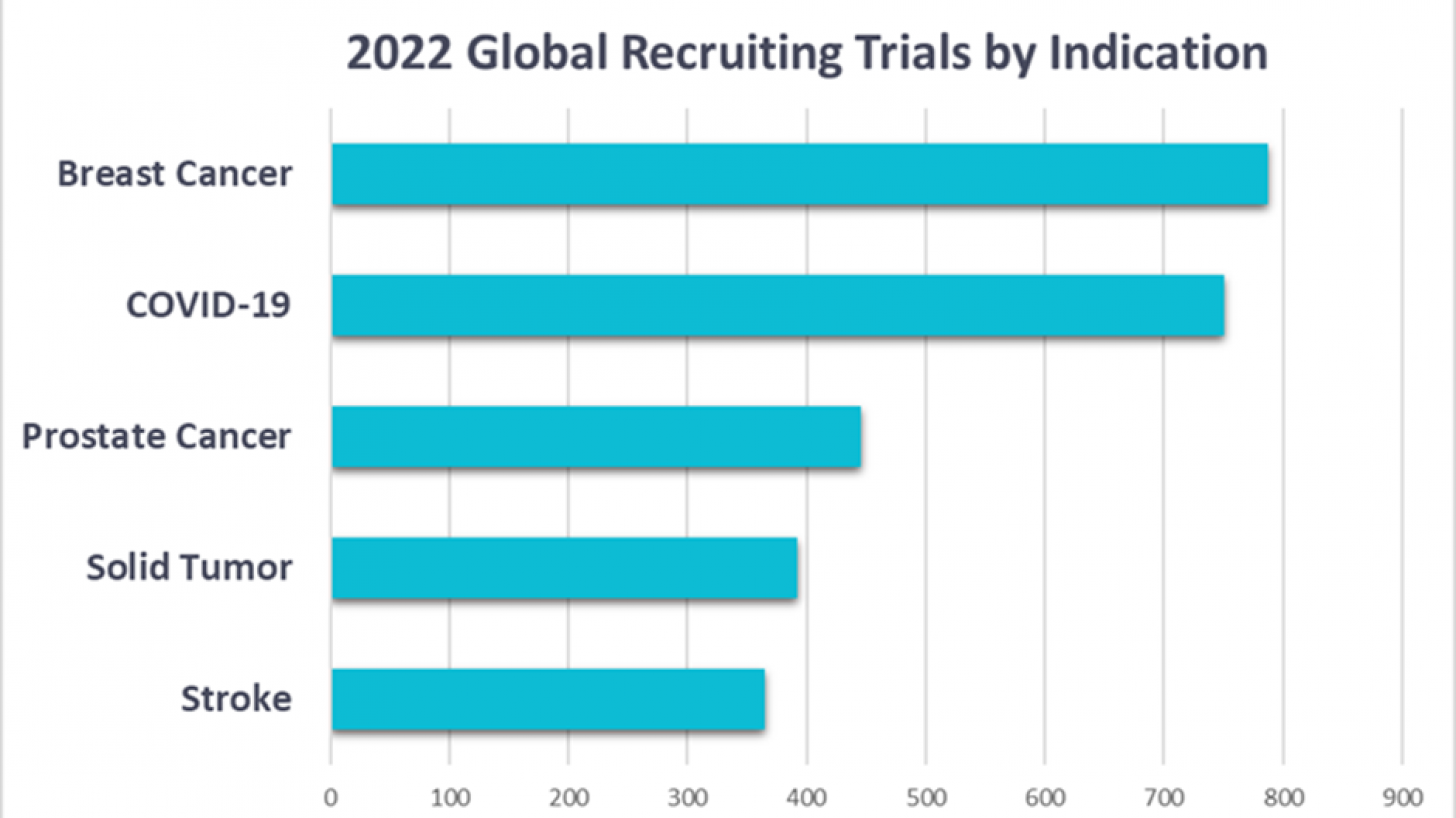
A global provider of patient-centric data analytics recently released the results of its global analysis of all clinical trials conducted in 2022. The new report, which analyzed over 80,000 records, revealed that breast cancer remains the most-studied disease.
Using Trial Accelerator™, Phesi disclosed in January 2023, that three of the top four most studied disease areas are within oncology, with prostate cancer as the third most studied disease and solid tumors fourth.
"Oncology remains an area of high investment in clinical development. However, 2022 has seen a widespread scaleback of overall activity. The reduction of recruiting trials in the top most-studied disease indications is due to several global factors, including the pandemic," commented Dr. Gen Li, President, Phesi, in a related press release on January 12, 2023.
"However, the reduction in breast cancer trials is unexpected, with 113 fewer recruiting trials in 2022 compared with 2021."
"This demonstrates the pressures facing the clinical development industry as the consequences from several years of disruption become visible."
The analysis has also revealed an increase in Phase II terminations.
In 2022, the attrition rate for Phase II clinical trials was 28% – 42% higher than the previous five-year average.
These high levels of attrition in Phase II will likely have a lasting effect on the clinical development industry. They may slow the rate at which new therapies reach the market or even prevent viable new therapies from ever reaching patients.
"As we enter the fourth year of the pandemic, the industry has more tools to mitigate its impact, but signs of damage continue to emerge. As a result, the clinical trial design must follow a more data-led, patient-centric approach to minimize protocol amendments and terminations and ensure successful study outcomes," commented Dr. Gen Li.
"Applying predictive analytics in protocol design, simulating trials, and using digital patient profiles will accelerate clinical development to get therapies to patients faster."
Phesi uses the world's largest clinical trial database to simulate clinical development and improve decision-making.
With AI-driven solutions for trial simulation, patient profiling, protocol design, and digital control arms are available to support life sciences companies in accelerating drug development and commercialization, says Phesi.
Our Trust Standards: Medical Advisory Committee