Cell-Based Flu Shot Effectiveness Surpass Egg-Produced Vaccines

Vaccines have proven to be the primary means of reducing influenza infections over decades. Although many people think all flu shots are the same, there are significant differences between cell—and egg-based vaccines, which provide different health benefits.
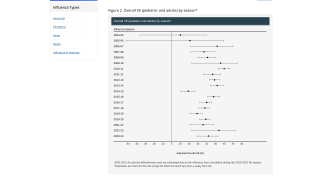
A real-world evidence study published on May 2, 2024, in Open Forum Infectious Diseases showed that a cell-based quadrivalent influenza vaccine (QIVc) was more effective at preventing test-confirmed cases of the flu than the egg-based quadrivalent influenza vaccine (QIVe) over three consecutive flu seasons in the United States (2017-2020).
These new findings show a higher relative effectiveness of QIVc over QIVe in the prevention of test-confirmed influenza, with estimated relative vaccine effectiveness (rVE) of 14.8% for the 2017–18 season, 12.5% for 2018–19, and 10.0% for 2019–20.
"This study exemplifies the value of RWE in generating valuable insights into the effectiveness of influenza vaccines," said Alicia N. Stein, Director, Real World Evidence, Center for Outcomes Research and Epidemiology, CSL Seqirus, in a press release.
Influenza vaccines produced using egg-based manufacturing can undergo adaptation within the manufacturing process, which may result in a mismatch between the flu strains covered in the vaccine and those identified by the World Health Organization (WHO).
Twice each year, the WHO selects flu stains for the next flu season's influenza vaccines.
"Cell-based technology allows us to provide an exact antigenic match to the WHO's identified strains, helping to improve vaccine effectiveness versus standard egg-based vaccines. These data build on the growing evidence of real-world data that shows the benefits of our cell-based influenza vaccine," added Gregg Sylvester, Chief Health Officer and Head of Medical Affairs at CSL Seqirus.
"At CSL Seqirus, we're continually pushing vaccine technology forward to improve effectiveness and help keep communities healthy."
The U.S. FDA's Vaccines and Related Biological Products Advisory Committee met on March 5, 2024, and finalized quadrivalent and trivalent influenza vaccine options for 2024-2025.
As of March 6, 2024, CSL Seqirus received the FDA's approval to produce its trivalent influenza vaccines.
Various flu shots should be available in U.S. pharmacies by August 2024.
As of April 6, 2024, an estimated 37 million flu shots were administered in retail pharmacies, and physicians' medical offices administered about 25 million doses.
Our Trust Standards: Medical Advisory Committee