Portfolio of Influenza Vaccines Shipped Ahead of 2024-2025 Flu Season

According to Stefan Merlo, Vice President of Commercial Operations, North America, CSL Seqirus, pharmacies in the United States will soon offer updated flu shots.
Mr. Merlo exclusively informed Precision Vaccinations News that as of July 9, 2024, CSL Seqirus was already shipping its differentiated influenza vaccines for the 2024/25 Northern Hemisphere flu season.
"This year, we've transitioned to trivalent vaccine formulations, aligning with U.S. FDA guidelines."
"Our primary goal is to bolster protection against influenza— a mission that grows more critical season over season as immunization rates decline. By ensuring timely and widespread distribution of our vaccines, we aim to support healthcare providers in safeguarding their patients and communities this flu season."
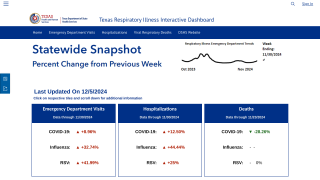
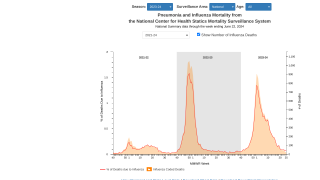
"The recent declines in flu vaccination rates pose a significant impact on public health, particularly by straining healthcare systems and resulting in the U.S. CDC seeing higher influenza-related hospitalizations and deaths compared to season’s past."
"This unfortunate trend was seen across various and vulnerable age groups, with a 22% drop among children aged six months to 18 years and a 14% decrease in adults over 65, a group that typically maintains stable immunization rates."
"In response to the decline in immunization rates, CSL Seqirus is actively enhancing awareness of this significant problem and collaborating with public health stakeholders across the country to meet and exceed pre-pandemic vaccination levels."
"With shipments underway, we are confident in our ability to deliver supply consistently and do our part to increase immunization rates and reduce the burden of influenza."
"At CSL Seqirus, we remain at the forefront of the fight against influenza, committed to offering influenza vaccine options and protecting communities from influenza-related complications."
"Our innovative and diverse influenza vaccine portfolio is specifically designed to meet the unique needs of different age groups, ensuring that all eligible individuals can safely receive their annual flu vaccination."
"This commitment not only protects individuals from influenza but also safeguards their loved ones by reducing the severity and spread of the virus," concluded Merlo.
CSL Seqirus offers a differentiated influenza vaccine option in the U.S. approved for use in people aged six months and older, which includes:
FLUCELVAX® is a cell-based influenza vaccine indicated for use in people six months and older.
FLUAD®, the first and only adjuvanted seasonal influenza vaccine for adults 65 and older, is preferentially recommended by the CDC Advisory Committee on Immunization Practices over standard-dose influenza vaccines. FLUAD contains an MF59® adjuvant designed to strengthen, broaden, and lengthen the immune response when added to an influenza vaccine.
AFLURIA® is an egg-based influenza vaccine approved for use in eligible people six months and older.
During the last flu season in the U.S., local pharmacies successfully administered a significant share of influenza vaccines. This trend is expected to continue in 2024-2025 since pharmacies offer appointment scheduling and the broadest selection of vaccines.
Our Trust Standards: Medical Advisory Committee