FDA Approves GamaSTAN Changes for Hep A and Measles

The Food and Drug Administration (FDA) has approved a new formulation of GamaSTAN® immune globulin (human) for hepatitis A virus (HAV) and measles post-exposure prophylaxis.
In addition to HAV and measles, GamaSTAN® is also approved by the FDA for rubella and varicella post-exposure prophylaxis.
GamaSTAN® is the only immune globulin product on the U.S. market approved for immediate protection against HAV and measles.
"Vaccination, while a valuable option for hepatitis A and measles post-exposure prophylaxis, may take several weeks to take effect as your immune system works to build the antibodies it needs to fight these viruses," said Dr. Stephen Scholand, Infectious Disease Specialist at MidState Medical Center.
"Immune globulins such as GamaSTAN® have been a valuable treatment option for many decades because they offer immediate and rapid protection with antibodies that fight infection," said Dr. Scholand.
The Centers for Disease Control and Prevention (CDC) states that for persons aged more than 40 years, an immune globulin is preferred for HAV post-exposure prophylaxis.
The CDC also recommends that immune globulin should be used for children aged less than 12 months, immunocompromised persons, persons with chronic liver disease, and persons who are allergic to the vaccine or a vaccine component.
When administered within 2 weeks after exposure to HAV, the immunoglobulin is 80 to 90 percent effective in preventing hepatitis A infection.
GamaSTAN® is available to U.S. healthcare providers in two vial sizes (10 mL and 2 mL).
Hepatitis A is an acute infectious disease of the liver caused by HAV.
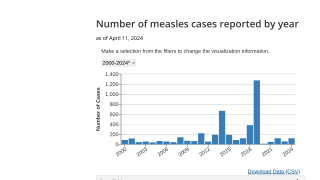
In 2018, the CDC estimates over 2,500 new cases of HAV in the U.S. across multiple states, which is on track to surpass the 2,984 cases in 2017.
Older people and people with a chronic liver disease, such as those infected with hepatitis C virus, are more likely to become seriously ill and die from HAV.
Measles is a highly contagious viral respiratory illness that is transmitted from person to person by direct contact with respiratory droplets or airborne spread and is the most contagious of all the vaccine-preventable diseases.
After exposure, up to 90 percent of susceptible persons develop measles, the deadliest of all childhood rash and fever illnesses.
GamaSTAN® is indicated for persons who have been exposed to measles fewer than 6 days previously and have not been vaccinated nor had measles.
Additionally, GamaSTAN® may be especially indicated for susceptible household contacts of measles patients, particularly children under 1 year of age, for whom the risk of complications is highest.
GamaSTAN® is not indicated for routine prophylaxis or treatment of viral hepatitis type B, rubella, poliomyelitis, mumps, or varicella.
Our Trust Standards: Medical Advisory Committee