Triple Punch Attenuated Malaria Helps in Vaccine Development

While current treatment methods can be effective against malaria, researchers across the world agree that an effective vaccine is needed to eliminate the disease completely.
Researchers believe vaccinations are the most promising approach to protect against malaria infection.
Currently, there is not a commercially available malaria vaccine.
But, there over 20 vaccine constructs being evaluated in clinical trials or are in advanced preclinical development.
In 2015, 91 countries and areas had ongoing malaria transmission. Between 2010 and 2015, malaria incidence among populations at risk (the rate of new cases) fell by 21% globally.
The good news is that malaria mortality rates among populations at risk fell by 29% globally among all age groups, and by 35% among children under 5.
In a recent study, researchers reveal that a vaccine called GAP3KO stimulated an effective immune response against the malaria parasite called Plasmodium falciparum.
"We had already good indicators in preclinical studies that this new 'triple knock-out' GAP (GAP3KO), which has three genes removed, is completely attenuated," says study co-author Sebastian Mikolajczak, Ph.D., principal scientist at the Center for Infectious Disease Research.
Dr. Robert Sedar, chief of cellular immunology at the Vaccine Research Center of the National Institutes of Health (NIH), said these findings are a "major advance in malaria vaccine development. Future studies demonstrating protective efficacy will be the next critical milestone for continued development of this promising vaccine approach."
Study co-author Dr. James Kublin, a scientist at the Fred Hutchinson Cancer Research Center in Seattle, WA, and medical director of the Seattle Malaria Clinical Trials Center, believes that the team's human malaria challenge model, whereby malaria vaccine candidates are tested in healthy adults, positions them for future research.
"We are very fortunate to have the human malaria challenge model to take the critical next step evaluating the efficacy of GAP3KO in preventing malaria in people," Dr. Kublin said.
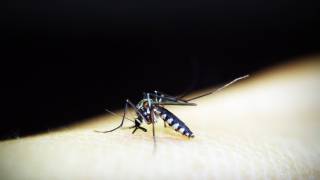
Malaria is a potentially fatal blood-borne disease caused by Plasmodium parasites, which are most commonly spread through the bites of female Anopheles mosquitoes. P. falciparum is the most widespread malaria parasite, and it is also one of the most deadly.
According to the World Health Organization (WHO), there were approximately 212 million new cases of malaria worldwide, and around 429,000 deaths from the disease, in 2015. More than 90 percent of malaria cases and deaths occur in sub-Saharan Africa.
Prevention strategies for malaria include insecticides, bed nets, and antimalarial drugs, while the primary treatment for the disease is artemisinin-based combination therapy.
Recent progress has been made with the completion of a Phase 3 trial of the RTS,S/AS01 candidate vaccine and review by the European Medicines Agency and WHO.
These research results warrant further clinical testing of Pf GAP3KO and its potential development into a vaccine strain.
These authors contributed equally to this work: James G. Kublin, Sebastian A. Mikolajczak, Brandon K. Sack, Matt E. Fishbaugher, Annette Seilie, Lisa Shelton, Tracie Von Goedert, Melike Firat and others. No conflicts of interest were disclosed.
Our Trust Standards: Medical Advisory Committee