mRNA Pancreatic Cancer Vaccine Candidate Shows Promising Results

Messenger RNA (mRNA) vaccines may be the hottest thing in vaccine research. But even before the recent pandemic began, Memorial Sloan Kettering Cancer Center (MSK) researchers had already been testing mRNA vaccine technology with a different purpose — as a treatment for cancer.
Pancreatic cancer surgeon Vinod Balachandran, MD, has been leading the first clinical trial to test mRNA vaccines as a potential therapy for pancreatic cancer.
The vaccines are custom-made for every person.
They use proteins in pancreatic tumors, called neoantigens, to alert the immune system that cancer cells are foreign.
The hope is that the vaccine will reduce the risk of cancer returning after the primary tumor is removed by surgery. The vaccines train the body to protect itself against its abnormal cancer cells.
As reported in Nature on May 10, 2023, this early-stage study suggests this innovative vaccine can cause an effective and lasting immune response.
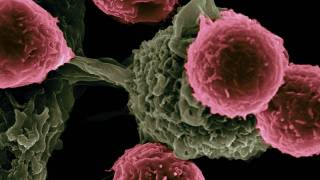
In 8 of 16 patients studied, the vaccines activated powerful immune cells, called T cells, that can recognize pancreatic cancer-specific to a patient.
These patients also showed delayed recurrence of their pancreatic cancers, suggesting that the T cells activated by the vaccines may have the desired effect — to keep pancreatic cancers in check.
“These exciting results indicate we may someday be able to use vaccines as a therapy against pancreatic cancer,” Dr. Balachandran says. “The evidence supports our strategy to tailor each vaccine to each patient’s tumor.”
The full, unedited MSK article is posted at this link.
This research is essential since pancreatic ductal adenocarcinoma is the third leading cause of cancer death in the U.S. and the seventh in the world, with an increasing incidence and a survival rate of 12% that has remained stagnant for nearly 60 years.
Our Trust Standards: Medical Advisory Committee