Therapeutic Vaccines Improve the Body’s Immune Response to HIV

On National HIV Vaccine Awareness Day 2019, a certain type of vaccine is being highlighted by the National Institutes of Health (NIH).
These ‘therapeutic’ vaccines don’t prevent diseases, they are designed to treat them.
A therapeutic HIV vaccine is a vaccine that’s designed to improve the body’s immune response to HIV in a person who already has HIV, says the NIH.
Currently, there are no therapeutic HIV vaccines approved by the Food and Drug Administration (FDA), but research is underway.
In the 2019 State of the Union Address earlier this year, President Trump announced the Administration’s goal to end the HIV epidemic in the United States within 10 years; Ending the HIV Epidemic: A Plan for America.
This Plan aims to reduce new HIV infections in the USA by 90 percent by 2030.
Researchers are developing and testing therapeutic HIV vaccines for the following reasons:
- to slow down the progression of HIV infection into AIDS
- to eliminate the need for antiretroviral therapy (ART) while still keeping undetectable levels of HIV
- as part of a larger strategy to eliminate all HIV from the body
In this regard, the NIH is pursuing a path to developing an HIV vaccine is based on theory and involves studying the body’s immune response to HIV infection and generating and enhancing those responses through vaccination.
The main theoretical approach to developing an HIV vaccine aims to prevent HIV infection by eliciting broadly neutralizing antibodies (bNAbs), which are antibodies shown in the laboratory to stop most HIV strains from infecting human cells.
Some people living with HIV naturally produce bNAbs. However, these antibodies develop too late after initial infection to clear the virus. Scientists at NIH and other institutions have isolated numerous bNAbs from people living with HIV and are working to develop vaccines that elicit these antibodies in healthy people.
Two experimental structure-based vaccines aimed at eliciting bNAbs directed against various components of the HIV envelope are in or near the early stages of human study.
A Phase 1 trial testing the BG505 SOSIP.664 gp140 trimer vaccine candidate is currently enrolling men and women in Boston; Seattle; and Nairobi, Kenya.
And, planning for a Phase 1 clinical trial to test a fusion peptide HIV vaccine developed by scientists at the NIAID Vaccine Research Center also is underway.
In addition to attempts to elicit antibodies to HIV via a vaccine, two multinational clinical trials are testing whether it is possible to prevent HIV by directly infusing people with bNAbs several times a year.
Known as the AMP Studies, for antibody-mediated prevention, these trials have completed enrollment of 4,600 men and women across four continents. If these studies prove successful, it will provide a rationale for using bNAbs as tools to prevent HIV infection.
In addition, it would provide the proof of concept that if vaccines induce these bNAbs, such vaccines would be successful in preventing HIV infection.
A list of clinical trials on therapeutic HIV vaccines is available from the AIDS info database of ClinicalTrials.gov:
- Comparison of Dendritic Cell-Based Therapeutic Vaccine Strategies for HIV Functional Cure - Recruiting
- Safety of and Immune Response to Vaccination With 2 Experimental HIV Vaccines in Healthy Adults - Unknown status
- Evaluating the Safety and Immune Response of an Adenovirus-Based HIV Vaccine in HIV-Uninfected Adults - Completed
- Safety and Immunogenicity Study of 2 Investigational Preventive HIV Vaccines - Completed
- Safety of and Immunogenicity to an H1N1 Influenza Vaccine in HIV-infected Adults - Completed
- Safety of and Immune Response to an Investigational HIV-1 Vaccine With or Without Interleukin-12 (IL-12) in HIV-1 Infected Adults - Completed
- Safety of and Immune Response to Two HIV Vaccines: SAAVI DNA-C2 Boosted With SAAVIMVA-C, in HIV-Negative Adults - Completed
- Safety and Immunogenicity Study of tgAAC09, a Gag-PR-RT AAV HIV Vaccine - Completed
- Therapeutic HIV Vaccine and Interleukin-2 to Increase the Immune System's Response to HIV - Completed
- Safety of and Immune Response to an Adenoviral HIV-1 Vaccine in Healthy Adults - Completed
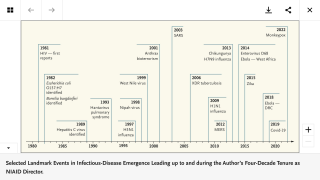
“While the ambitious Plan for America aims to end HIV as an epidemic within the United States in 10 years, achieving a durable end to the pandemic will almost certainly require a safe and effective HIV vaccine,” said Anthony S. Fauci, M.D., Director, National Institute of Allergy and Infectious Diseases.
“On this HIV Vaccine Awareness Day, we recognize and thank the thousands of HIV vaccine clinical trial volunteers, researchers, health professionals, activists, and others who work with us toward this goal,” said Dr. Fauci in a press release on May 18, 2019.
Learn more about a promising method for fighting HIV by visiting this NIH page.
Our Trust Standards: Medical Advisory Committee