Yellow Fever Vaccine Availability Continues In 2020

To help meet the continued yellow fever vaccine consumer demand in the USA, the manufacturer of the STAMARIL® vaccine announced it will continue to be available throughout 2020.
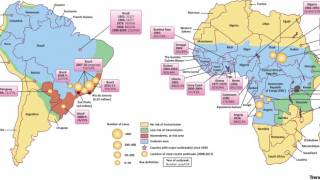
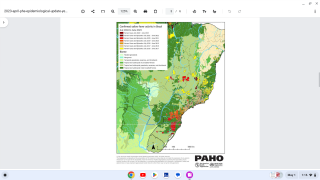
This is good news since numerous countries around the world have continued to report yellow fever outbreaks during 2019.
And several countries require international visitors to prove they have already been vaccinated against yellow fever prior to entering their country.
Moreover, there are no approved medicines to treat or cure an infection.
Sanofi Pasteur said in a statement distributed on December 23, 2019, it has worked with the US Food and Drug Administration (FDA) to provide the STAMARIL vaccine through an Expanded Access Program (EAP), during the YF-VAX vaccine shortage.
This EAP allows for the importation and use of the STAMARIL vaccine in the USA in place of the YF-VAX vaccine until the production of YF-VAX vaccine resumes to meet consumer demand.
Sanofi reaffirmed in this press statement they are making every effort to see that yellow fever vaccination continues in the USA during this YF-VAX vaccine supply disruption.
More than 250 locations throughout the USA have already been selected to be included in the STAMARIL EAP.
STAMARIL remains investigational/unlicensed in the USA but is currently approved for distribution in over 70 countries.
Sanofi Pasteur continued saying ‘Since 2016, it has recognized the importance of yellow fever vaccination to protect people and has been working with FDA and other stakeholders to ensure the STAMARIL vaccine remains available for travelers, U.S. government employees, military, and other response groups during the anticipated shortage period.'
"The availability of yellow fever is necessary for the freedom of travel of Americans to many countries," said Crockett Tidwell RPh, CDE, United Supermarkets Pharmacy.
"Not only does it offer protection against this deadly disease, but is often a legal requirement."
"Those of us chosen as Stamaril vaccine providers will continue to fill this vital role until the return of YF-Vax," said Crockett.
Vaccine Overviews
- Stamaril is a live, attenuated yellow fever vaccine that contains the active substance Yellow fever virus1 17D-204 strain produced in specified pathogen-free chick embryos. Stamaril is indicated for active immunization against yellow fever for adults and children aged 9 months and older.
- YF-VAX is prepared by culturing the 17D-204 strain of yellow fever virus in living avian leukosis virus-free (ALV-free) chicken embryos. The vaccine contains sorbitol and gelatin as a stabilizer, is lyophilized, and is hermetically sealed under nitrogen. YF-VAX is indicated for active immunization for the prevention of yellow fever in persons 9 months of age and older.
Sanofi also renders a consumer website that offers vaccine financial support information for prospective, qualifying customers.
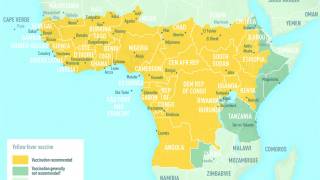
Healthcare providers and patients may also visit for information about which countries require yellow fever vaccination for entry and for which countries the Centers for Disease Control and Prevention (CDC) recommends yellow fever vaccination.
The CDC said in May 2019 ‘a safe and effective yellow fever vaccine has been available for more than 80 years. The vaccine is a live, weakened form of the virus, given as a single shot.’
Additionally, the CDC says ‘the Yellow fever vaccine has been given to many pregnant women without any apparent adverse effects on the fetus. However, since the yellow fever vaccine is a live virus vaccine, it poses a theoretical risk.’
While a 2-week delay between yellow fever vaccination and conception is probably adequate, a 1-month delay has been advocated as a more conservative approach, says the CDC.
Yellow fever vaccine news
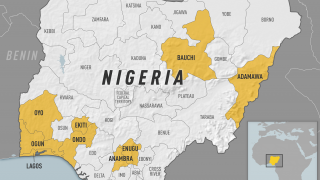
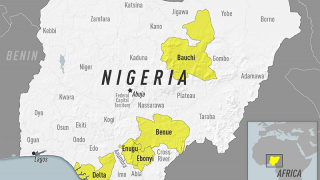
- Yellow Fever Fatalities Reach Alert-Level in Nigeria
- Level 4 Travel Advisory Updated for the Bolivarian Republic of Venezuela
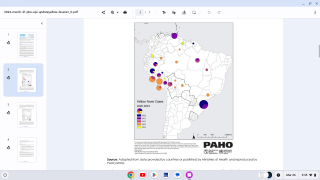
- Brazil Executives Testing The USA’s Global Entry System
- Health Alerts For Travelers During Winter 2019
- Andes Mountain Border Areas Launch Vaccination Campaign
If for some reason a woman is vaccinated during pregnancy, she is unlikely to have any problems from the vaccine and her baby is very likely to be born healthy.
And, ‘pregnant women should avoid or postpone travel to an area where there is a risk of yellow fever. If travel cannot be avoided, discuss vaccination with your healthcare provider prior to departure,’ says the CDC.
The yellow fever virus is spread to people by the bite of an infected mosquito. Illness ranges from a fever with aches and pains to severe liver disease with bleeding and yellowing skin (jaundice).
Yellow fever infection is diagnosed based on laboratory testing, a person’s symptoms, and travel history.
> Online lab tests at UltaLabs <
Yellow fever vaccine news published by Vax-Before-Travel
Our Trust Standards: Medical Advisory Committee