West Nile Virus Vaccines
West Nile Virus Vaccine Candidates
As of October 2024, no U.S. Food and Drug Administration (FDA)-approved West Nile virus (WNV) vaccines are available for people. However, active human clinical studies include several vaccine candidates: two live attenuated chimeric, one DNA, one recombinant subunit, and two inactivated whole-virus vaccines.
In phase 2 trials, the live attenuated recombinant yellow fever vaccine strain expressing the premembrane and envelope (prM–E) genes of WNV (ChimeriVax-WN02, Sanofi Pasteur) was found to have a good safety profile and immunogenicity even in older age groups after a single dose.
The HydroVax-001 vaccine candidate consists of a hydrogen peroxide inactivated whole virion (WNV-Kunjin strain) adjuvanted with aluminum hydroxide.
"Our experience over the past two decades has demonstrated that current prevention strategies are insufficient to reduce the ongoing WNV disease burden. WNV vaccination would be more effective in preventing WNV disease and related deaths," wrote a Perspective published by the NEJM on May 4, 2023. "Although serious adverse events were not reported in early clinical trials, potential safety concerns must be addressed in future WNV vaccine development efforts. The benefits of live vaccines, including durability of immunity and the need for only one dose, will need to be weighed against potential safety concerns."
West Nile Virus Outbreaks
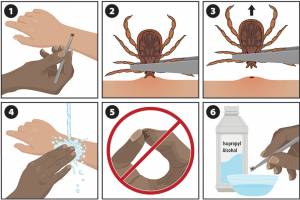
Since 1999, West Nile Virus (WNV) has become the leading cause of arthropod-borne viral (arboviral) disease in the United States, says the U.S. Centers for Disease Control and Prevention (CDC). In Europe, the European Centre for Disease Prevention and Control (ECDC) shows that there have been 715 locally acquired cases of WNV across 15 countries in Europe in 2024, surpassing the number reported in the same period in 2023 and Europe's 10-year average. As of September 2024, 51 people had died as a result of the infection. A study published in the Journal of Experimental Medicine on June 22, 2023, found that about 35% of patients hospitalized for WNV also carried autoantibodies that neutralize type 1 interferons, the signaling proteins deployed by various cells in the fight against viruses.