Positive Phase 2 Trial Data Targeting Advanced HPV-Positive Cancers

PDS Biotechnology Corporation announced yesterday expanded interim data in the Phase 2 clinical trial investigating the PDS0101-based triple combination therapy in advanced human papillomavirus (HPV)-positive cancers.
The interim efficacy data from 37 HPV16-positive evaluable patients, including 29 patients in the checkpoint inhibitor (CPI) refractory arm, are consistent with the results presented at ASCO 2022 and affirm the selection of CPI refractory patients as the initial patient population for ongoing clinical development of the triple combination.
The NCI-led Phase 2 clinical trial is investigating PDS0101 in combination with two investigational immune-modulating agents – M9241, a tumor-targeting IL-12 (immunocytokine), and bintrafusp alfa, a bifunctional checkpoint inhibitor (PD-L1/ TGF-β) – in recurrent or metastatic HPV-positive cancers in patients who have failed prior therapy.
The triple combination is being studied in CPI-naïve and -refractory patients with advanced HPV-positive anal, cervical, head and neck, vaginal, and vulvar cancers.
Highlights of the expanded interim data are as follows:
- Survival data: 66% (19/29) of HPV 16-positive CPI refractory patients in the cohort were alive at a median follow-up of 16 months. Historically, this group has a median overall survival of only 3-4 months.
- Safety profile: 48% (24/50) of patients experienced Grade 3 treatment-related adverse events (AEs), and 4% (2/50) patients experienced Grade 4 AEs. There were no grade 5 treatment-related AEs.
- Results for HPV 16-positive checkpoint inhibitor naïve patients also continue to appear to be encouraging: 75% (6/8) of CPI naïve patients were alive at a median of 25 months of follow-up. In addition, 38% (3/8) of responders had a complete response.
“The expanded interim data investigating the PDS0101-based triple combination therapy in advanced HPV-positive cancers continue to appear to show clinical signs of efficacy, durability, and safety in an extremely challenging patient population with very few available treatment options,” stated Dr. Frank Bedu-Addo, President and CEO of PDS Biotech, in a press release on October 11, 2022.
“Importantly, these results affirm the decision to explore this novel combination for the treatment of CPI refractory patients, who have no approved standard of care, and support the development of a combination therapy to address the significant unmet need.”
This Phase 2 study is being conducted at the Center for Cancer Research at the National Cancer Institute (NCI).
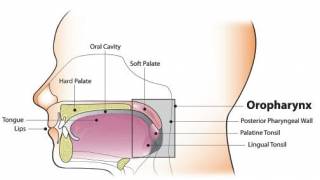
The NCI says HPV is a group of more than 200 related viruses. Sexually transmitted HPV types fall into two groups, low-risk and high-risk.
High-risk HPV infections that persist can cause cancer. When a high-risk HPV infection persists for many years, it can lead to cell changes that, if untreated, may get worse over time and become cancer.
M9241 and bintrafusp alfa are owned by Merck KGaA, Darmstadt, Germany.
Our Trust Standards: Medical Advisory Committee