Ten Years Later, HPV Vaccination Remains Very Protective

After the introducing of Human Papillomavirus (HPV) vaccines in 2006, real-world studies have demonstrated decreases in the prevalence of specific HPV types and reduced rates of cervical lesions and cancer in vaccinated populations, wrote the American Academy of Pediatrics (AAP).
On September 5, 2023, the AAP stated the accumulated safety data from extensive postmarketing surveillance and epidemiologic studies conducted from 2009 through 2021 across 13 countries have been consistent with the safety profile in clinical trials.
These post-licensure results and the results from long-term follow-up extensions of clinical trials, including the study described herein, continue to support the favorable benefit-risk profile of HPV vaccination.
The complete study is posted at this AAP link.
"These data highlight the importance of GARDASIL 9® in the prevention of certain HPV-related cancers and diseases later in life," said Dr. Eliav Barr, senior vice president, head of global clinical development, and chief medical officer, Merck Research Laboratories, in a Merck press release on September 5, 2023.
"HPV-related cancers and diseases are a significant public health issue. These strong study results serve as a reminder that we need to do everything we can to expand and recover vaccination rates globally to help protect all eligible people from certain HPV-related cancers."
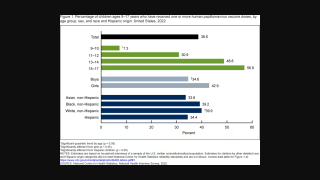
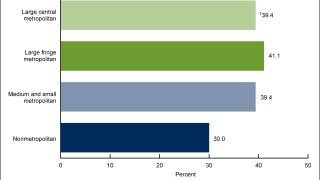
This is essential cancer prevention news since HPV will infect most sexually active males and females in their lifetime, writes the U.S. CDC.
For most people, there is no way to know who has HPV, except for cervical cancer, as there is no routinely recommended screening to detect HPV-related cancers.
GARDASIL 9 targets HPV types that cause approximately 80% of cervical cancers. Additionally, HPV is responsible for causing genital warts and certain types of vulvar, vaginal, and anal cancers.
For most people, HPV, a sexually transmitted disease, clears on its own, but it could lead to certain cancers and other diseases for those who don't clear the virus.
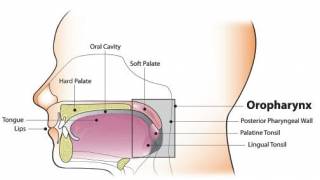
In the U.S., GARDASIL 9 is indicated for use in females 9 through 45 years of age for the prevention of cervical, vulvar, vaginal, anal, oropharyngeal and other head and neck cancers caused by HPV Types 16, 18, 31, 33, 45, 52, and 58; cervical, vulvar, vaginal, and anal precancerous or dysplastic lesions caused by HPV Types 6, 11, 16, 18, 31, 33, 45, 52, and 58; and genital warts caused by HPV Types 6 and 11.
GARDASIL 9 is also indicated for use in males 9 through 45 years of age for the prevention of anal, oropharyngeal, and other head and neck cancers caused by HPV Types 16, 18, 31, 33, 45, 52, and 58; anal precancerous or dysplastic lesions caused by HPV Types 6, 11, 16, 18, 31, 33, 45, 52, and 58; and genital warts caused by HPV Types 6 and 11.
The oropharyngeal and head and neck cancer indication is approved under accelerated approval based on its effectiveness in preventing HPV-related anogenital disease. Continued approval for this indication may be contingent upon verification and description of clinical benefit in a confirmatory trial. The confirmatory trial is ongoing.
GARDASIL 9 is contraindicated in individuals with hypersensitivity, including severe allergic reactions to yeast, or after a previous dose of GARDASIL 9 or GARDASIL® [Human Papillomavirus Quadrivalent (Types 6, 11, 16, and 18) Vaccine, Recombinant].
Globally, there are several authorized HPV vaccines.
Disclosure: Funding for this research was provided by Merck Sharp and Dohme LLC, a subsidiary of Merck and Co, Inc., Rahway, NJ.
Our Trust Standards: Medical Advisory Committee