Lyme Disease Preventative Oral, Non-Vaccine Candidate Progress
Tarsus Pharmaceuticals, Inc. today announced positive topline results from the completed Phase 1b Callisto and the first subject's enrollment in the Carpo Phase 2a clinical trials.
These human-based studies evaluate the novel investigative oral therapeutic TP-05 for the potential prevention of Lyme disease.
TP-05 is an oral systemic formulation of lotilaner, a well-characterized anti-parasitic agent that selectively inhibits parasite-specific GABA-CI channels.
TP-05 is believed to be the only non-vaccine, drug-based, preventative therapeutic in development designed to kill ticks to potentially prevent Lyme disease transmission.
"Lyme disease is a growing global health concern that may result in serious, often irreversible sequelae if left untreated," said José Trevejo, MD, Ph.D., Chief Medical Officer of Tarsus, in a press release on December 15, 2022.
"Tarsus is committed to addressing large, underserved disease areas with unique solutions, and our TP-05 investigational program embodies this commitment."
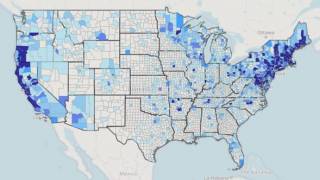
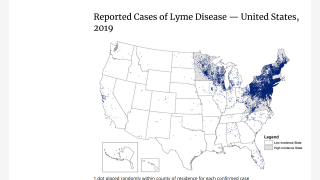
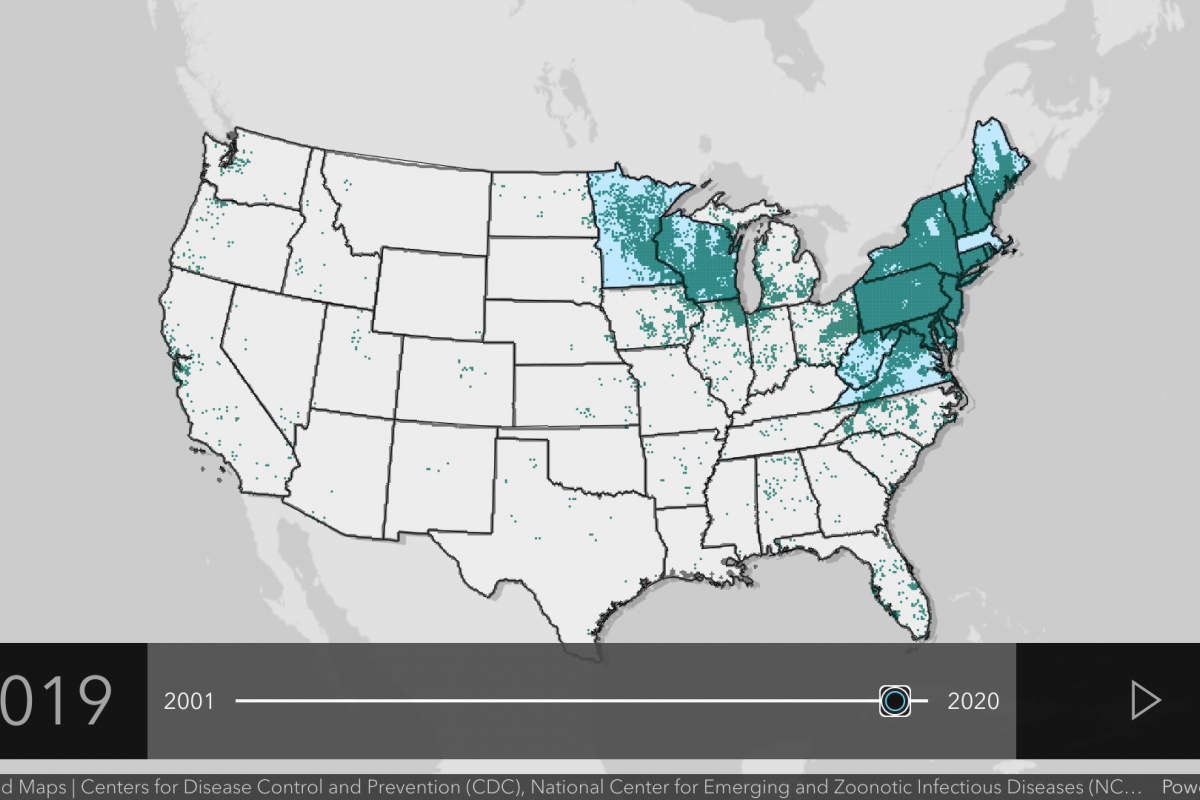
Lyme disease is the most common vector-borne disease in the U.S.
A recent report from FAIR Health indicates Lyme diagnoses rose 357% in rural regions and 65% in urban areas from 2007 to 2021.
About 30 million U.S. residents are at risk of contracting Lyme disease.
While no U.S. Food and Drug Administration (FDA) Lyme disease (LD) vaccines are approved today, Pfizer Inc. and Valneva SE's co-developed VLA15 vaccine candidate is conducting late-stage phase 3 studies.
The innovative VLA15 vaccine confers protection by raising antibodies that prevent Borrelia from migrating from ticks to humans after a bite.
The VLA15 vaccine development program was granted Fast Track designation by the U.S. FDA in July 2017.
Our Trust Standards: Medical Advisory Committee