Influenza and mRNA COVID-19 Vaccine Combo Posts Positive Topline Data

Pfizer Inc. and BioNTech SE today announced positive topline results from a Phase 1/2 clinical study evaluating the safety, tolerability, and immunogenicity of mRNA-based combination vaccine candidates for influenza and COVID-19 among healthy adults.
The data from the trial showed that the companies' lead formulations demonstrated robust immune responses to influenza A, influenza B, and SARS-CoV-2 strains.
The topline results of the ongoing trial demonstrated that the combination formulations evaluated had a safety profile consistent with the safety profile of the companies' COVID-19 vaccine.
In the Phase 1/2 clinical trial, the vaccine candidates were compared to a licensed influenza vaccine and the companies' Omicron BA.4/BA.5-adapted bivalent COVID-19 vaccine given at the same visit.
A pivotal Phase 3 trial evaluating these lead formulations is expected to be initiated in the coming months.
"Studies of confirmed viral infections suggest that COVID-19 adopts a seasonal pattern with peaks in fall and winter, similar to other respiratory diseases. Co-infections and consecutive respiratory infection during this period can further increase the risk of severe illness," said Prof. Ugur Sahin, MD, CEO and Co-founder of BioNTech, in a press release on October 26, 2023.
"Combination vaccines have the potential to become a mainstay of routine vaccination against respiratory diseases, especially for the vaccination of populations with a higher risk of severe illness."
Immunogenicity results induced by lead formulations in the companies' phase 1/2 trial showed point estimates for Geometric Mean Titer (GMT) ratios consistent with the criteria applied to regulatory-approved vaccines against the respective influenza and SARS-CoV-2 strains.
Point estimates for GMT ratios for all matched influenza vaccine strains with lead formulations were >1 relative to a licensed Quadrivalent Influenza Vaccine given concomitantly with the Pfizer-BioNTech COVID-19 vaccine.
Pfizer and BioNTech previously announced that their mRNA-based combination vaccine candidate for influenza and COVID-19 received Fast Track Designation from the U.S. Food and Drug Administration.
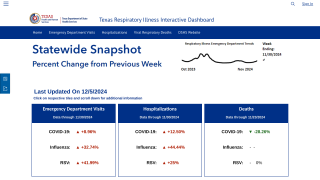
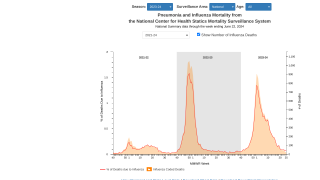
For the 2023-2024 flu season in the U.S., 136.94 million influenza vaccine doses had been distributed as of October 14, 2023.
Our Trust Standards: Medical Advisory Committee