68% of Parents Eagerly Await Lyme Disease Vaccines

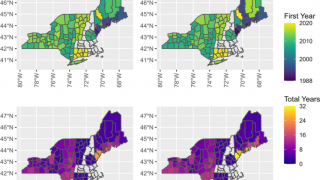
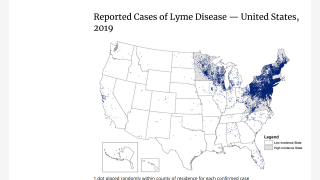
A new survey published in the journal Vaccine revealed about 68% of parents in high- and emerging-incidence states would vaccinate their children against Lyme disease.
With Lyme disease vaccines approaching approval, there appears to be significant consumer demand in some U.S. states.
Announced on February 28, 2024, this survey found addressing safety concerns important, and a healthcare provider recommendation could also encourage those unsure or unwilling to be vaccinated.
Given the slight preference for monoclonal antibody passive immunization over-vaccination, particularly in rural areas, access to both may increase Lyme disease prevention.
Initially developed by Valneva SE, the VLA15 Lyme disease vaccine candidate development program was granted Fast Track designation by the U.S. Food and Drug Administration in 2017.
The journal The Lancet Infectious Diseases published results from a Jully 2023 study that concluded Valneva's novel multivalent Lyme vaccine candidate was safe and immunogenic.
VLA15 is currently in advanced phase 3 clinical study.
Our Trust Standards: Medical Advisory Committee