Brazil Produced Dengue Vaccine May Arrive in 2024

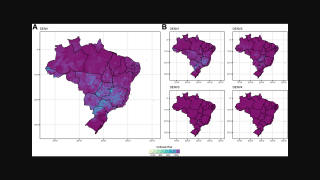
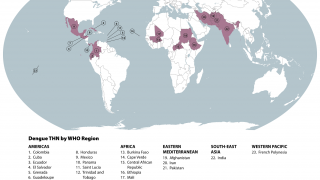
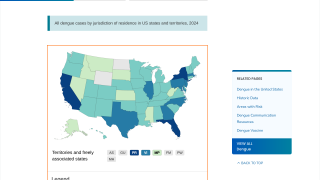
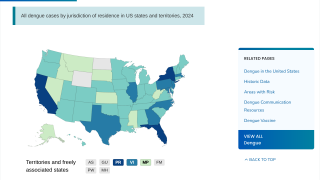
It has become clear that Brazil is currently facing its largest dengue fever outbreak. While two dengue vaccines have been approved for use in the country, each one has its own set of issues.
As of April 12, 2024, vaccine producers are focused on protecting more Brazilians against the virus over the next year.
Sanofi Pasteur's Dengvaxia vaccine is meant for individuals aged 9-45 who already have dengue. It requires three doses and pre-administration testing.
On the other hand, Takeda's second-generation QDENGA® (TAK-003) two-dose vaccine is also approved for use, but it has already sold out its production for 2024.
As of January 2024, the Ministry of Health forecasted that 5.2 million doses will be delivered in 2024. Unfortunately, that amount will leave millions of people unprotected this year.
Furthermore, an article published by The New England Journal of Medicine in January 2024 stated that the Butantan-DV single-dose vaccine candidate offers protection against all four dengue virus serotypes without regard to dengue baseline serostatus and across a wide age range.
In addition to the logistical and economic benefits, Butantan-DV rapid protection may be necessary if Brazil's dengue outbreak accelerates.
The development of this novel tetravalent dengue vaccine began at Butantan Institute in 2010, using a formulation created by researchers affiliated with the U.S. NIH.
Based on recent phase 3 clinical trial results, Butantan Institute plans to submit a report to ANVISA in 2024, applying for the vaccine's registration.
"The cost of dengue in Brazil is absurd," virologist Maurício Lacerda Nogueira said in a press release in February 2024.
"The (Butantan-DV) vaccine is expected to reduce mortality and hospitalizations due to the disease, so the Brazilian government's investment of several hundred million reais in developing an indigenous vaccine will have a huge impact on public health."
Our Trust Standards: Medical Advisory Committee