Second Generation Dengue Vaccine Produces Durable T-Cell Protection

Every virologist says creating robust cellular responses is essential for protecting people against infectious diseases. While vaccine immunogenicity evaluations often focus predominantly on antibody responses, generating cellular immunity is crucial for long-term protection.
To appreciate the benefits of any vaccination, measuring T-cell responses is essential, especially for disease outbreaks such as dengue fever, which has four sub-viruses (DENV-1, -2, -3, and -4).
A recent phase 2 study evaluated adolescents' kinetics and phenotype of T cell responses induced following vaccination with QDENGA® (TAK-003), a second-generation, live-attenuated tetravalent dengue vaccine.
Published by the journal NJP, this study concluded that TAK-003 was well tolerated and elicited durable T-cell responses against all four DENV serotypes irrespective of baseline serostatus.
Furthermore, TAK-003 elicited multifunctional CD4 and CD8 T cells and persisted up to three years post-vaccination.
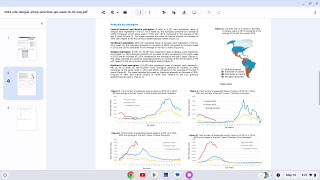
In both dengue-naïve and previously-exposed participants, TAK-003 elicited a strong and rapid T-cell response, with over 90% of subjects achieving a positive response within a month of receiving the first dose.
Positive responses were seen across all four serotypes.
A subgroup analysis of participants 10 years and older showed that T-cell responses were evident within 14 days of vaccination.
This study's findings contrast with the analysis of T-cell responses to the only other licensed dengue vaccine, the first-generation CYD-TDV (Dengvaxia). Unlike TAK-003, which uses a DENV-2 backbone, CYD-TDV is based on a yellow fever YF-17D backbone.
Although the TAK-003 backbone includes only DENV-2-specific NS1, NS3, and NS5 proteins, conservation of these proteins across DENV serotypes likely resulted in simultaneous induction of T cell responses against DENV-1, -3, and -4 following vaccination with TAK-003.
This durable, multitypic T cell immunity may contribute to the prevention of severe dengue in TAK-003 vaccinees and is consistent with the vaccine's long-term efficacy and safety profile, where no increase in severity of disease has been observed in vaccinees with breakthrough infections along with durable high level of protection against dengue leading to hospitalization.
These findings have global significance in preventing additional dengue outbreaks, which may be vastly underreported.
Published by The Lancet Infectious Diseases on October 25, 2024, Bos and colleagues showed that mild or inapparent dengue infections are even more frequent (88%) than previously thought.
And that DENV serotypes differ in the extent of clinical symptoms.
DENV3 was more likely to cause symptomatic and even severe primary dengue than DENV1 and DENV2, while DENV2 had the highest rate of inapparent primary infections.
In the United States, 50 jurisdictions, led by California, Florida, New Jersey, New York, and Puerto Rico, had reported 6,819 travel-related and locally acquired dengue cases as of October 31, 2024. In Florida, DENV-3 and 4 are the most often detected viruses.
While unavailable in the U.S., the QDENGA vaccine sales significantly increased in numerous countries in 2024.
Our Trust Standards: Medical Advisory Committee