Second-Generation Dengue Vaccine Delivers Protection in Two Weeks

International travelers booking last-minute trips to disease-outbreak countries often wonder how quickly their vaccination will provide protection. A new study has shown that in just two weeks, Takeda Vaccines, Inc.'s second-generation dengue vaccine protects people from this mosquito-transmitted disease.
This finding is great news for many travelers, as the global dengue outbreak continues unabated in September 2024.
This year, 43 countries and territories in the Region of the Americas have reported over 11.5 million dengue cases and about 6,478 related deaths. As of September 2024, this Pan American Health Organization data is over 125% greater than for all of 2023.
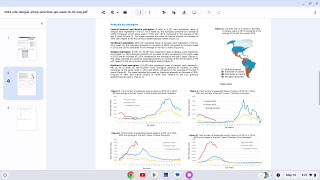
Furthermore, the DEN-301 clinical trial concluded that QDENGA® (TAK-003) tetravalent, two-dose (at 0 and 3 months) vaccine efficacy (VE) against virologically confirmed dengue exceeds 82%.
This Short Communication published in the journal Vaccine on September 7, 2024, disclosed the VE between the first and second Qdenga doses was consistent with data observed after both doses in both the safety population (80.9 % [95 % CI: 75.1–85.3]) and the per-protocol set (80.2 [95 % CI: 73.3–85.3]).
This research concluded, 'This (data) indicates that the vaccine may be useful in a dengue outbreak or as a travel vaccine, where the rapid onset of protection before administration of the second dose of the approved two-dose schedule is desirable.'
From an availability perspective, the World Health Organization added QDENGA to its List of Prequalified Vaccines in May 2024. This means most countries can order this dengue vaccine.
Over the past year, 40 countries have approved Qdenga, and 24 have launched or made this innovative vaccine available to their populations.
According to previous media announcements, the plan for Qdenga to become available in the U.S. is being evaluated, given the immediate need for those living in dengue-endemic areas, such as Puerto Rico and Florida.
Disclosure: This study was sponsored by Takeda Vaccines, Inc. Takeda was involved in the study design, data collection, analysis, and interpretation, writing the manuscript, and deciding to submit the article for publication.
Our Trust Standards: Medical Advisory Committee