Dengue Vaccines Harder to Find in 2024

During the U.S. Centers for Disease Control and Prevention (CDC) vaccine committee meeting this afternoon, it will be mentioned that finding a dengue vaccine in 2024 poses a challenge for international travelers.
At 5 PM ET on June 26, 2024, the CDC's Advisory Committee on Immunization Practices (ACIP) meeting is scheduled to review two presentations that reveal the leading dengue vaccines are unavailable this year to U.S.-based international travelers.
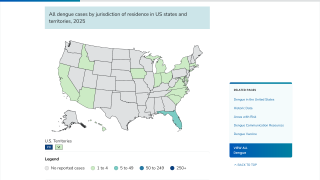
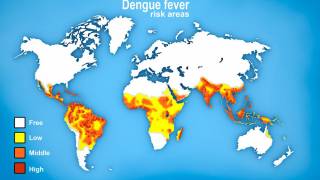
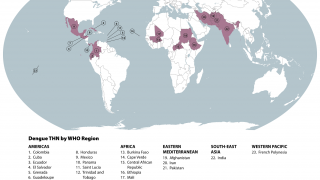
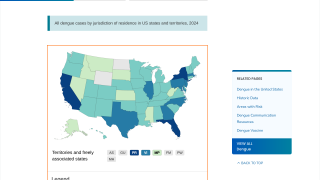
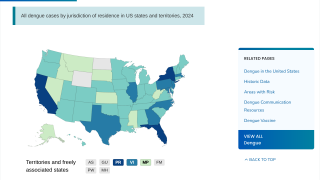
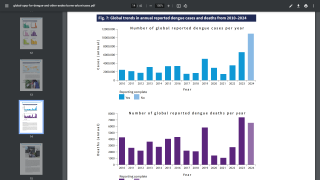
This is unfortunate news for people planning to visit any of the 90 countries reporting dengue cases in 2024.
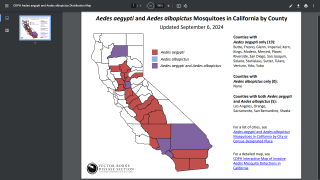
Furthermore, the CDC says dengue is endemic in six U.S. territories and freely associated states. This health risk includes Puerto Rico and southeast Florida, where the CDC has reported travel-related and locally acquired dengue cases in 2024.
Dengvaxia®, the only U.S. FDA-approved dengue vaccine, is discontinued due to low demand. According to Sanofi, the vaccine's producer, this decision was not based on quality, safety, or efficacy concerns.
Since Dengvaxia vaccination began in Puerto Rico, 145 people have been administered the vaccine, but only 32 have completed the third dose of the series.
Dengvaxia will continue to be distributed globally through public and private markets, including Puerto Rico, where the ACIP currently recommends it until the product expires. The last doses of Dengvaxia will expire at the end of August 2026.
Over the past year, Japan's Takeda's Qdenga® vaccine became the market leader, quickly selling out its production capability. In July 2023, Takeda voluntarily withdrew Qdenga from FDA review, which means the vaccine is unavailable in the U.S.
The good news is that various dengue vaccine candidates are conducting late-stage clinical trials in 2024, with aspirations for market authorizations later this year or in 2025.
Our Trust Standards: Medical Advisory Committee