Second-Gen Dengue Vaccine Found in 24 Countries
Recently, there has been confusion for international travelers looking to protect themselves from dengue disease, which is transmitted to people by mosquitoes.
In January 2024, Sanofi-Pasteur announced that they would stop manufacturing the U.S. FDA-approved Dengvaxia® vaccine for children due to a lack of demand in the global market.
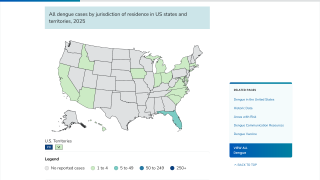
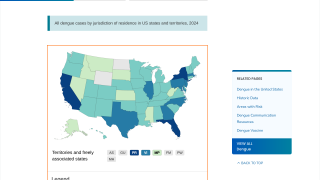
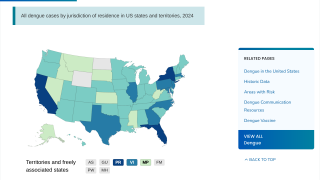
As a result, Dengvaxia is no longer available in the United States, except in Puerto Rico, where dengue has become endemic.
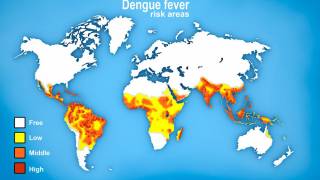
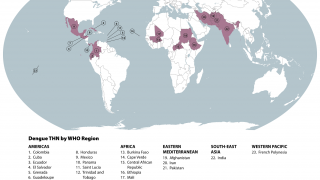
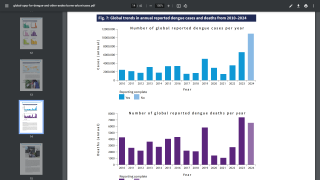
Then, the global dengue outbreak accelerated in the Region of the Americas.
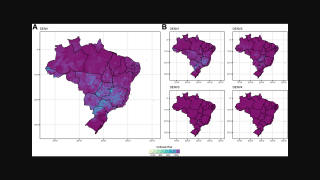
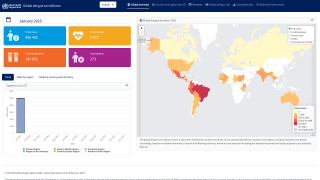
As of July 25, 2024, 43 countries and territories in the Region had reported over 10.8 million Dengue cases and about 5,848 related deaths. This data is over 100% greater than recorded throughout 2023.
Recently, the U.S. Centers for Disease Control and Prevention (CDC) reissued its Global Travel Health Notice, notifying healthcare providers, public health authorities, and the public of an increased risk of dengue virus infections in the U.S. in 2024.
However, the CDC could not recommend an alternative to the Dengvaxia dengue vaccine.
But during last October's CDC's vaccine committee meeting, the Takeda QDENGA® Dengue Tetravalent Vaccine was presented, reviewing its efficacy in preventing dengue fever and/or Severe Dengue.
In addition to offering people protection, QDENGA does not require pre-admission testing.
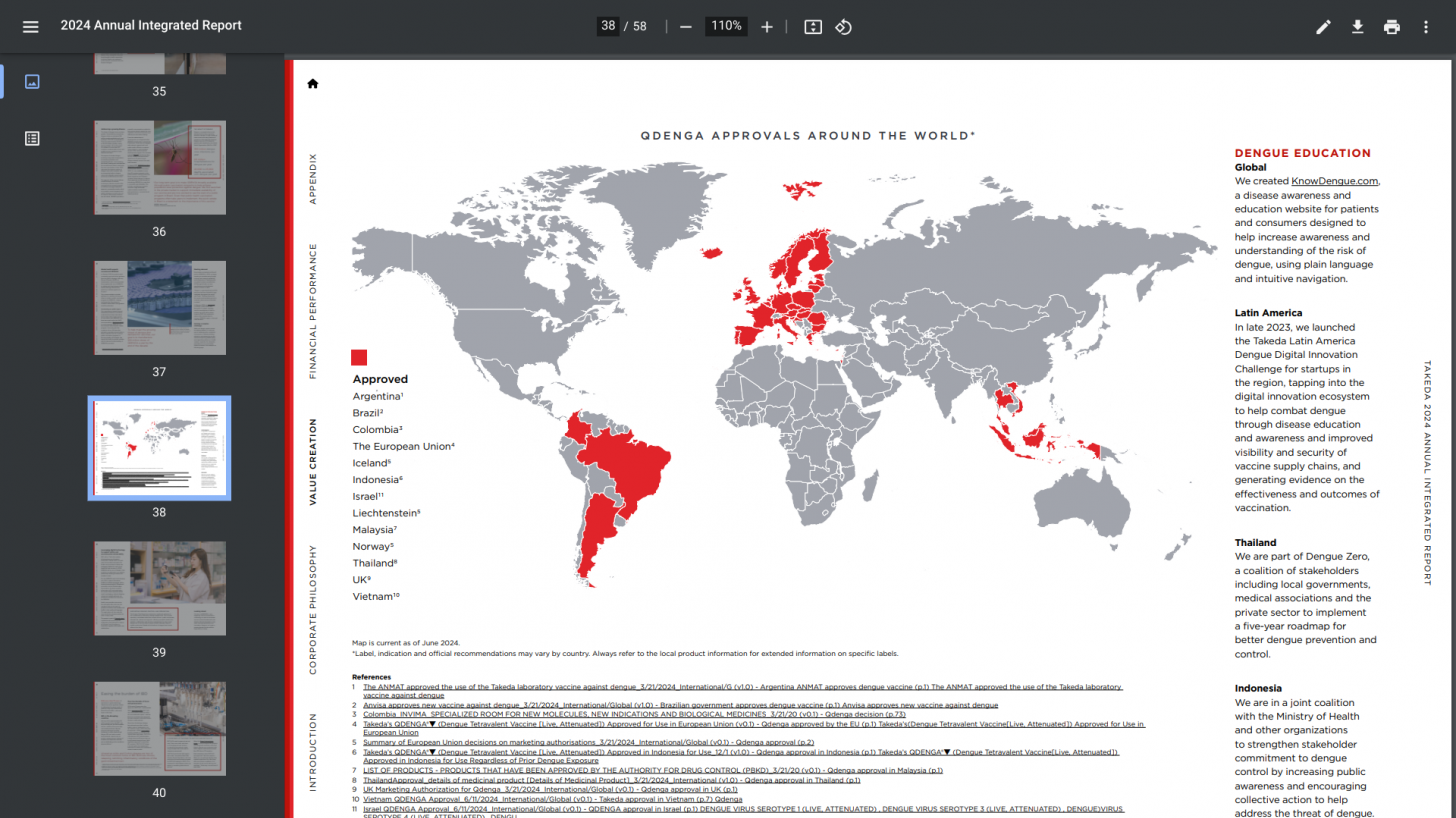
While QDENGA is not U.S. FDA-approved, many international travelers wonder where to obtain this second-generation, two-dose vaccine.
As of late July 2024, Takada revealed that QDENGA is available in private markets in 24 countries: Indonesia (approved Aug 2022) (available April 2023), Brazil (approved Mar 2023) (available June 2023, Thailand (approved May 2023) (available Aug 2023), Argentina (approved Apr 2023) (available Oct 2023), Malaysia (approved Feb 2024) (available June 2024), Colombia (approved Sept 2023) (available July 2024), Germany (approved Dec 2022) (available Feb 2023), Finland (approved Dec 2022) (available Feb 2023), Sweden (approved Dec 2022) (available Feb 2023), Norway (approved Dec 2022) (available Feb 2023), Denmark (approved Dec 2022) (available Mar 2023), The Netherlands (approved Dec 2022) (available Mar 2023), Luxembourg (approved Dec 2022) (available March 2023), Czech Republic (approved Dec 2022) (available April 2023), Austria (approved Dec 2022) (available April 2023), Belgium (approved Dec 2022) (available April 2023), Ireland (approved Dec 2022) (available April 2023), Portugal (approved Dec 2022) (available May 2023), Spain (approved Dec 2022) (available May 2023), U.K. (approved Jan 2023) (available July 2023), Slovakia (approved Dec 2022) (available Aug 2023), Italy (approved Dec 2022) (available Oct 2023), Poland (approved Dec 2022) (available Dec 2023), Israel (approved May 2024) (available July 2024).
Furthermore, Takeda recently announced that it is expanding its production capabilities to meet the growing global demand for QDENGA.
Biological E. Limited committed in February 2024 to manufacturing up to 50 million QDENGA vaccine doses annually, accelerating Takeda's ability to deliver 100 million doses annually by 2030.
As this vaccine becomes more available, the CDC suggests the best way to avoid dengue is to prevent mosquito bites when visiting endemic areas, such as Brazil.
Our Trust Standards: Medical Advisory Committee