Third Dengue Vaccine Arriving Soon

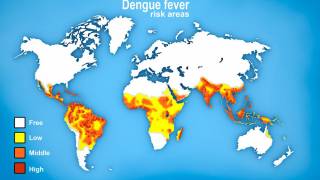
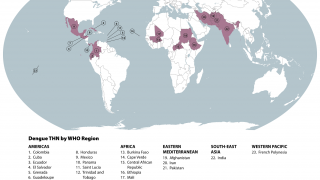
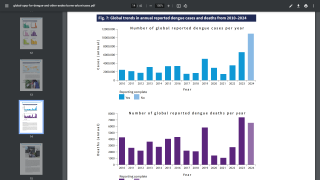
While numerous countries are confronting dengue fever outbreaks in 2024, with over 11 million cases in the Region of the Americas, there is a pending shift in accessing preventive vaccines.
As of early August 2024, the World Health Organization recommends using a second-generation dengue vaccine: Takeda's QDENGA vaccine. However, there is overwhelming demand, surpassing current vaccine production capabilities.
Below is a Commentary published by The Lancet Infectious Diseases on August 5, 2024. It discusses how a dengue vaccine that is equally immunogenic and effective against all four viral serotypes would address one of the top ten public health needs.
In 2015, Dengvaxia (CYD-TDV; Sanofi Pasteur, Paris, France) was licensed, followed by the second vaccine, Qdenga (TAK-003; Takeda, Tokyo, Japan), in 2022.
A third dengue vaccine, the Butantan-Dengue Vaccine (Butantan-DV), seems next. Like its predecessors, Butantan-DV is a cocktail of four live-attenuated dengue viruses (DENVs).
However, three of the four dengue serotypes are near full-length DENV genomes, whereas only one component (DENV-2) is a chimeric vaccine virus.
This chimeric virus contains two structural genes of DENV-2 on the same attenuated DENV-4 genetic background as the DENV-4 component of the vaccine.
Consequently, Butantan-DV contains more homologous immunogenic dengue proteins for each serotype in the cocktail (including non-structural proteins for three of the four serotypes) than the other two highly chimeric vaccines.
Additionally, Butantan-DV requires only a single dose, unlike three doses for Dengvaxia and two doses for Qdenga. This makes Butantan-DV more amenable to programmatic uptake and for soon-departing travellers.
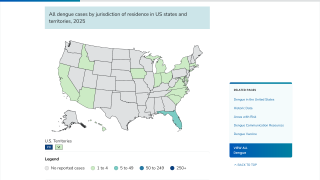
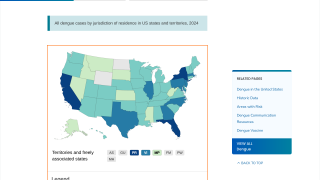
As of August 10, 2024, access to dengue vaccines in the United States is limited.
Our Trust Standards: Medical Advisory Committee