Next-Gen Pertussis Vaccine Candidate Proceeds to Phase III Clinical Trials

ILiAD Biotechnologies, LLC announced the selection of Emmes Group to conduct upcoming Phase III studies of its lead pertussis vaccine candidate, BPZE1.
As of June 24, 2024, ILiAD and Emmes Group are working to finalize the definitive agreement.
Multiple Phase III studies are expected to be conducted in North America, Central and South America, the U.K., and other global clinical sites.
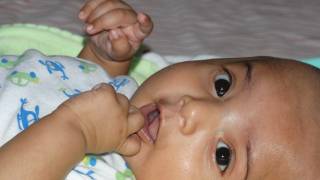
BPZE1 is the leading next-generation pertussis vaccine designed to induce comprehensive and durable protection against B. pertussis infection (colonization) and disease (whooping cough). This vaccine is being developed to block B. pertussis from colonizing the nasal passages of adults and children, to protect adults and children from whooping cough, and to potentially prevent transmission, including transmission to infants.
"We are honored and pleased that ILiAD has selected Emmes Group as its partner to continue the clinical development of BPZE1. We look forward to working closely with ILiAD's clinical development team on this promising new vaccine, which could significantly reduce the transmissibility and incidence of B. pertussis, particularly in vulnerable populations," said Sastry Chilukuri, Chief Executive Officer of Emmes Group, in a press release.
While ILiAD is currently focused on developing a vaccine to protect adults and children and indirectly protect vulnerable infants, future development aims to immunize neonates directly. BPZE1 was developed at the Institut Pasteur de Lille (France) in the lab of Camille Locht, PhD and Nathalie Mielcarek, PhD.
According to the U.S. CDC, reported pertussis cases in 2024 increased across the U.S., indicating a return to more typical trends. Preliminary data show that more than three times as many cases have been reported to date in 2024 compared to the same time in 2023.
Our Trust Standards: Medical Advisory Committee