New UTI Treatments Confirm Positive Advances in 2024

For the first time in decades, novel urinary tract infection (UTI) treatments and vaccines are making significant progress worldwide.
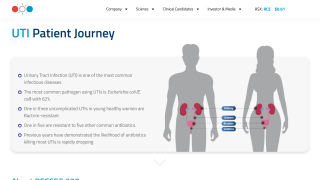
Recce Pharmaceuticals Ltd today announced positive data from a Phase I/II clinical trial for UTIs and urosepsis, demonstrating that RECCE® 327 (R327) administered intravenously is safe and efficacious against Escherichia coli (E. coli).
The trial data revealed on June 28, 2024, that R327's effect on bacterial growth was sustained over time. Significant activity was noted within the initial 0 to 45-minute window and extending to 2 to 4 hours post-dosing.
This extended period of activity suggests that R327 maintains its efficacy for prolonged durations, potentially enhancing its therapeutic value in clinical settings.
Recce also confirmed it is on track to initiate a Phase II trial of R327 with UTI patients later in 2024.
Dr. Marc Sharp, chief scientific officer at Linnaeus Bioscience and one of the leading independent experts in bacterial Mechanism of Action analysis, commented in a press release, "The ability of R327 to achieve biologically relevant concentrations and exhibit antibacterial activity in urine samples is highly encouraging."
This good news follows RECCE® 327's recent addition to the World Health Organization’s Antibacterial Agents in Clinical and Preclinical Development list.
And in the United States, the Food and Drug Administration (FDA) approved Pivya (pivmecillinam) on April 24, 2024, for adult women with uncomplicated UTIs.
The U.S. NIH says that compared to complicated UTIs, uncomplicated ones are less severe and more prevalent, affecting more than half of women during their lifetime.
Peter Kim, M.D., M.S., director of the Division of Anti-Infectives in the FDA’s Center for Drug Evaluation and Research, commented in a press release, “The FDA is committed to fostering new antibiotic availability when they prove to be safe and effective, and Pivya will provide an additional treatment option for uncomplicated UTIs.”
According to an NYT report, Pivya's availability in the U.S. is forecasted for 2025.
Furthermore, progress is being reported on Spain-based Immunotek S.L.'s Uromune™ (MV140) inactivated, oral spray UTI vaccine. Various clinical studies have evaluated the beneficial role of Uromen in preventing recurring UTIs.
Uromune began clinical evaluations in 2010 and is now available in Mexico, the Dominican Republic, Singapore, and England.
Off-license use is limited in about 20 countries through special access programs. The U.S. recently discontinued its Uromune vaccine access program.
Our Trust Standards: Medical Advisory Committee