UTI Treatment Options Now Include Antibiotics, Cranberries, Vaccines

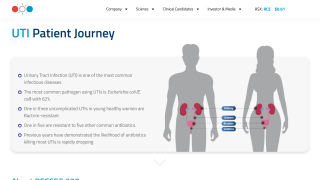
With over 50% of women reporting at least one urinary tract infection (UTI) in their lives, it has become a challenge for many women to identify the best interventions.
Most UTIs are bacterial bladder infections in females with no structural abnormalities of their urinary tract.
Based on recent announcements in 2024, efforts are being made to review science-based evidence supporting potential UTI interventions.
For example, a meta-analysis and systematic review of six studies published on July 18, 2024, offered evidence supporting possible nondrug preventions. This study found that antibiotic use was 49% lower with the consumption of cranberry juice than with placebo liquid and 59% lower with no treatment.
This study also found cranberry compounds were associated with a decrease in the prevalence of UTI symptoms.
Regarding new, innovative antibiotics, the U.S. Food and Drug Administration approved Pivya™ (pivmecillinam) tablets on April 24, 2024, for the treatment of female adults with uncomplicated UTIs caused by susceptible isolates of Escherichia coli, Proteus mirabilis, and Staphylococcus saprophyticus.
The FDA says Pivua is a unique class of antibiotics with potent in vitro and in vivo activity against the most common bacteria in UTI, including resistant strains such as ESBL-producing E coli.
"Uncomplicated UTIs are a prevalent condition impacting women and one of the most frequent reasons for antibiotic use," said Peter Kim, M.D., M.S., director of the Division of Anti-Infectives in the FDA's Center for Drug Evaluation and Research, in a press release.
Over the past decade, limited studies in Europe and other countries have tested UTI vaccines, revealing positive results.
Uro-Vaxom® (OM-89) is an immunostimulant vaccine consisting of an oral tablet with 18 heat-killed strains of E coli bacteria. A review found that the risk ratio for developing at least one UTI in the female population was significantly lower in the OM-89 group (RR 0.61, 95% CI 0.48–0.78), and the mean number of UTIs was about half compared to placebo.
Uro-Vaxom has been used in Europe with minimal documented adverse events and is recommended by the European Association of Urology.
Separately, Uromune™ (MV140) is an inactivated, oral spray, mucosal-based bacterial vaccine that reduces recurrent UTIs. In clinical research over the past few years, Uromune induced immune responses systemically and in the genitourinary tract, especially the bladder's innate immune system.
Since 2017, about 21,000 patients, representing 1.5 million doses of Uromune, have been administered in special access programs in various countries.
When selecting the best UTI therapy in 2024, availability becomes essential.
According to recent announcements, UTI vaccines and Pivya may become available in the United States in 2025, which leaves cranberry UTI therapy available today.
As with all clinical sessions, the U.S. CDC says speaking with a healthcare provider is the best option.
Our Trust Standards: Medical Advisory Committee