England Offers Ample Access to UTI Vaccines

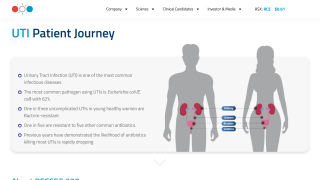
The United Kingdom's NHS recently updated its urinary tract infection (UTI) webpage to explain that some people may experience persistent UTI symptoms, and current urine tests may not detect chronic infections caused by bacteria embedded in the bladder lining.
Chronic UTIs, also known as recurrent UTIs (rUTIs), are typically treated with antibiotics. The NHS advises patients to ask their GP to refer them to a specialist if they experience rUTIs.
A recent study in England revealed that a novel oral spray vaccine could potentially prevent rUTIs for years. Researchers at the Royal Berkshire Hospital conducted a significant clinical trial using the Uromune® (MV140) vaccine.
Nine years after treatment, 54% of participants in the study reported the vaccine's continued efficacy without significant side effects.
In previous clinical research, Uromune induced immune responses systemically and in the genitourinary tract, especially the bladder's innate immune system.
This multi-dose vaccine contains four whole-cell inactivated bacteria: Escherichia coli, Klebsiella pneumoniae, Proteus vulgaris, and Enterococcus faecalis. Uropathogenic Escherichia coli accounts for the majority of community-acquired UTIs.
Recently, access to Uromune in England has expanded to patients who have been treated in secondary care before referral and have failed standard treatment protocols for uncomplicated urinary tract infections, overactive bladder, and chronic bladder pain.
These vaccine providers include:
London Urologica operates from The London Clinic, a charitable hospital in central London. At The London Clinic, international consultants with teams of health professionals specialize in the care and treatment of complex medical conditions.
The Lower Urinary Tract Symptoms Service is a specialist for adult patients with complex chronic or rUTIs. Specialists in this tertiary clinic adhere to protocols requiring strict clinical governance.
The Urology Partnership (The Reading Urology Partnership) comprises consultants, each with a particular field of excellence. All consultants hold substantive appointments at the Royal Berkshire NHS Foundation Trust Hospital in Reading, Berkshire.
For rUTI patients willing to travel, the Uromune vaccine may be offered via Expanded Access Programs. These programs are for individuals for whom antibiotic therapy has failed, antibiotic-induced adverse reactions, and increasing antibiotic resistance.
Uromune is offered in the Dominican Republic, Singapore, and other countries. However, Uromune is commercially unavailable in the United States and is not U.S. FDA-approved, but UTI vaccine clinical trials are active in 2024.
International vaccine appointments can be requested at Vax-Before-Travel.
Updated on July 16, 2024 to clarify the UTI vaccine is not U.S. FDA-approved.
Our Trust Standards: Medical Advisory Committee