Next-generation Oral Polio Vaccine Prequalified to Combat Outbreaks

Biological E. Limited (BE) today announced that the World Health Organisation (WHO) has granted Pre-qualification (PQ) status to their Novel Oral Polio Vaccine type 2 (nOPV2).
In collaboration with PT Bio Farma (PTB) in Indonesia, the first manufacturer of the nOPV2 vaccine to receive WHO Pre-Qualification in January 2024, BE has successfully received technology from PTB and qualified to produce more than 500 million doses of nOPV2 vaccine annually.
As of July 2024, over 1 billion nOPV2 vaccine doses have been administered.
BE has been approved by the Indian regulatory authorities to manufacture the vaccine for export purposes.
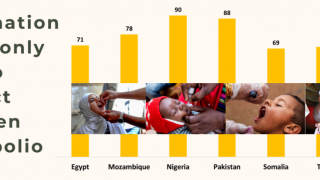
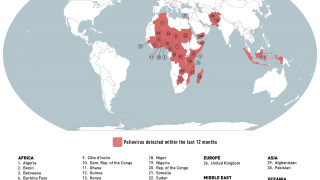
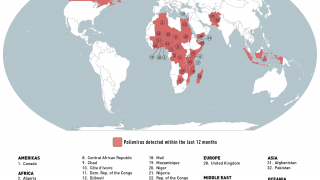
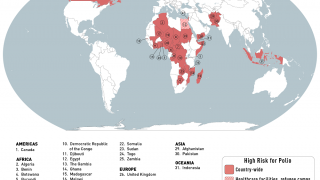
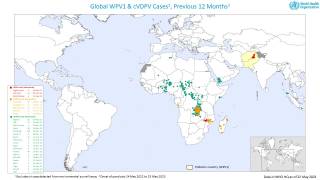
This next-generation live, attenuated oral polio vaccine significantly reduces the risk of circulating vaccine-derived Poliovirus type 2 (cVDPV2) outbreaks, which continues in various countries in 2024.
With its improved genetic stability, nOPV2 has a significantly decreased chance of seeding new outbreaks in low-immunity environments.
Furthermore, nOPV2's real-world deployment in outbreak regions has shown that it can significantly decrease the incidence of cVDPV2 outbreaks, safeguarding communities from the ravages of polio.
Ms. Mahima Datla, Managing Director, BE, said in a press release on July 30, 2024, "This vaccine has been specifically designed to address concerns about Vaccine-Associated Paralytic Polio, which has occurred in approximately 2 to 4 cases per million births with the traditional oral vaccine due to the vaccine virus reverting to a virulent form."
Ms. Datla further expressed BE's gratitude for the collaboration with PTB and the support of a grant from the Gates Foundation, “The significance of this milestone extends beyond scientific achievement; it represents a beacon of hope for millions of children and families around the globe."
Since 2000, the IPV vaccine has been available in the U.S.
In late 2023, the U.S. CDC published updated recommendations for using the IPV vaccine. Fully vaccinated adults at increased risk for poliovirus exposure may receive a single lifetime booster dose of IPV.
BE is a Hyderabad-based Pharmaceuticals & Biologics Company founded in 1953 and is the first private-sector biological products company in India.
Our Trust Standards: Medical Advisory Committee