U.S. Government Funds Advanced Ebola Treatment Candidate
RedHill Biopharma today announced that it had received a contract with the U.S. Biomedical Advanced Research and Development Authority (BARDA) to advance the development of opaganib, a small-molecule treatment for Ebolavirus.
This novel, potentially broad-acting drug has shown mutation-resistant antiviral and anti-inflammatory activity, likely to counteract the vascular impacts of Ebola infection.
In a press release on October 14, 2024, the company stated that it is pursuing an animal-rule pathway for potential approval for this Ebola treatment candidate. This process is used when human clinical trials are not ethical or feasible.
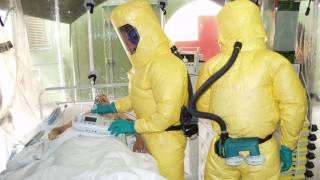
Guy Goldberg, RedHill's Chief Business Officer, commented, "Currently, only Inmazeb™, a combination of three monoclonal antibodies, and Ebanga™, a single monoclonal antibody, are FDA-approved to treat Ebola infections. As such, there is an urgent need for additional effective and easy-to-distribute and administer therapies (during an outbreak)."
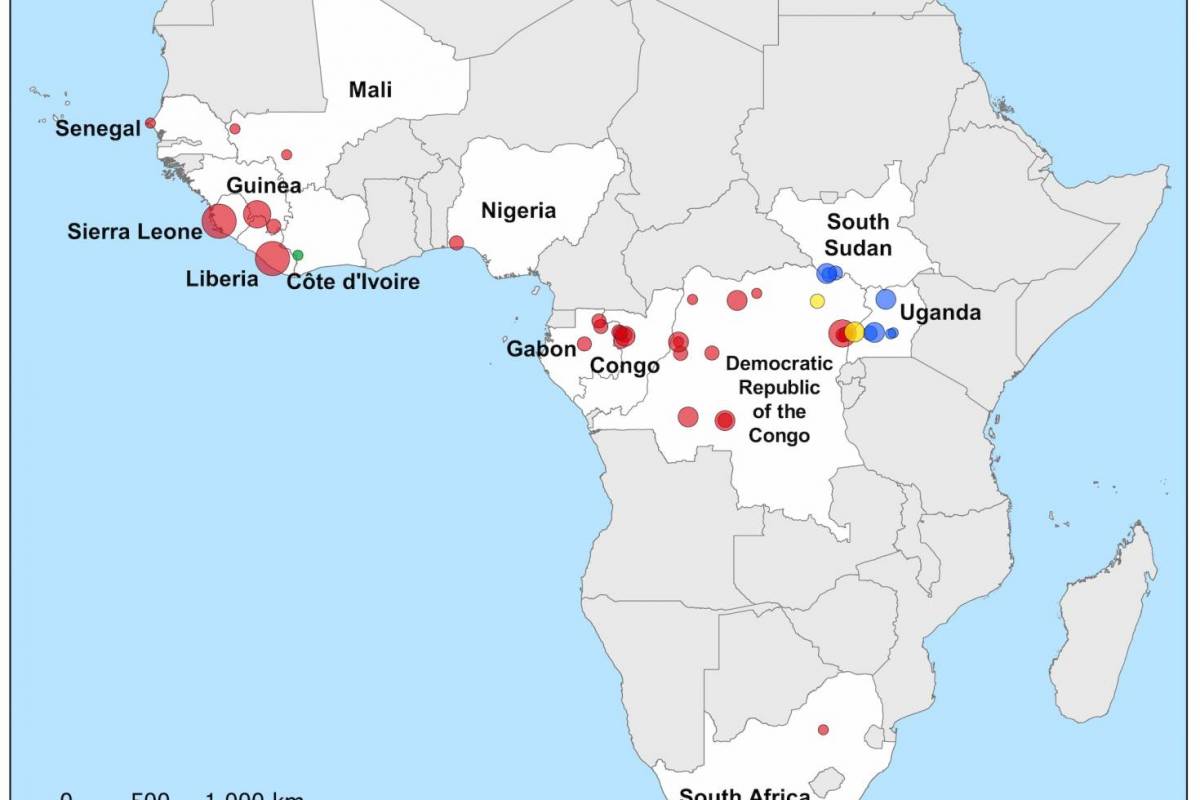
While there are approved Zaire Ebola vaccines and therapeutics available in 2024, previous outbreaks have highlighted significant logistical challenges that exist in managing Ebola outbreaks.
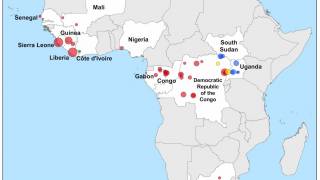
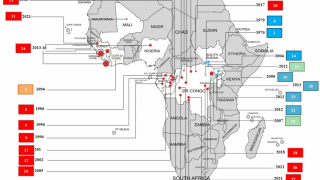
As of October 2024, more than 30 Ebola outbreaks have been reported in Africa. The initial Zaire Ebolavirus case was confirmed in 1976 in a village near the Ebola River in Africa, and the virus's origins remain enigmatic in 2024.
Our Trust Standards: Medical Advisory Committee