Adjuvanted and High-Dose Influenza Vaccines Offer Comparable Protection for Seniors

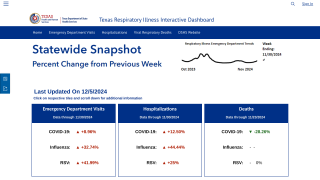
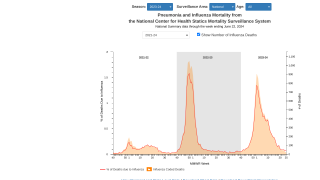
As the 2024-2025 flu season ramps up in the United States, new data reaffirms the protections offered by various influenza vaccines.
The results of the first pragmatic randomized study to evaluate the relative vaccine effectiveness of adjuvanted inactivated influenza vaccine (aIIV) compared to high-dose inactivated influenza vaccine (HD-IIV) for influenza prevention were published today.
The first season's results demonstrated that adjuvanted and high-dose influenza vaccines did not differ in effectiveness against Polymerase Chain Reaction-confirmed influenza with relative vaccine effectiveness of aIIV vs. HD-IIV of 1.5% (95% confidence interval: -8.4 to 10.5) among adults 65 years of age and older.
In addition, there was no difference in effectiveness between aIIV and HD-IIV for preventing hospitalization or emergency department visits for confirmed influenza and hospitalization for all-cause community-acquired pneumonia.
The data was presented during an oral presentation this week at the IDWeek 2024 conference.
The second season of the study is ongoing. Results may differ depending on the match between the vaccine and circulating influenza strains and other factors.
This study was funded by CSL Seqirus, which provided all the adjuvanted influenza vaccine doses to members of a large health system.
As of October 18, 2024, over 92 million flu shots have been distributed in the U.S. this season.
Our Trust Standards: Medical Advisory Committee