Bird Flu Vaccines for Birds Launch Clinical Studies
The United States Department of Agriculture (USDA) recently announced it launched avian influenza vaccination clinic trials with animals in April 2023 to help mitigate the latest outbreak of highly pathogenic avian influenza (HPAI) in the United States.
The USDA's Agricultural Research Service (ARS) published Fact Sheet Release No. 0084.23, which confirmed researchers are currently testing several vaccine candidates.
As of April 14, 2023, the initial data from an animal study with a single dose of the vaccine are expected to be available in May 2023. And these researchers expect to have two-dose vaccine challenge studies with results in June 2023.
Should the trials be successful, and should USDA elect to continue vaccine development, the next step is identifying manufacturers interested in vaccine production.
Once one or more manufacturers are identified, there are 20 discrete stages to complete before vaccine delivery. These stages begin with feasibility work by the manufacturer and culminate with product label submission and review.
In a best-case scenario, the USDA estimates an 18-24 month timeline before having a vaccine that matches the currently circulating avian virus strain, is available in commercial quantities, and can be easily administered to commercial poultry.
However, manufacturers may expedite development in emergencies, resulting in a shortened timeframe for licensure.
"Since the first case of HPAI was confirmed in a commercial flock in the U.S. in Feb. 2022, the USDA has followed Secretary Vilsack's clear direction to identify cases and respond immediately to stop the virus from spreading," commented Acting Deputy Secretary Kevin Shea in a press release.
"Thanks to collaborative state and industry partnerships and enhanced national animal disease preparedness and response capabilities, we are successfully controlling this outbreak and mitigating its impact on U.S. poultry production and trade."
Many factors make implementing a vaccine strategy challenging, from vaccine development to production timelines to dissemination to flocks. It would take time to deliver an effective vaccine, wrote the USDA.
Marketing and Regulatory Programs Under Secretary Jenny Moffitt added, "All of these lessons learned have informed our current strategy of stamping out and eradicating HPAI – which continues to be the most effective strategy because it works."
"For example, during the 2014-2015 outbreak, 70% of HPAI cases were attributed to lateral spread."
"Whereas in this outbreak, the lateral spread has been reduced to 15%."
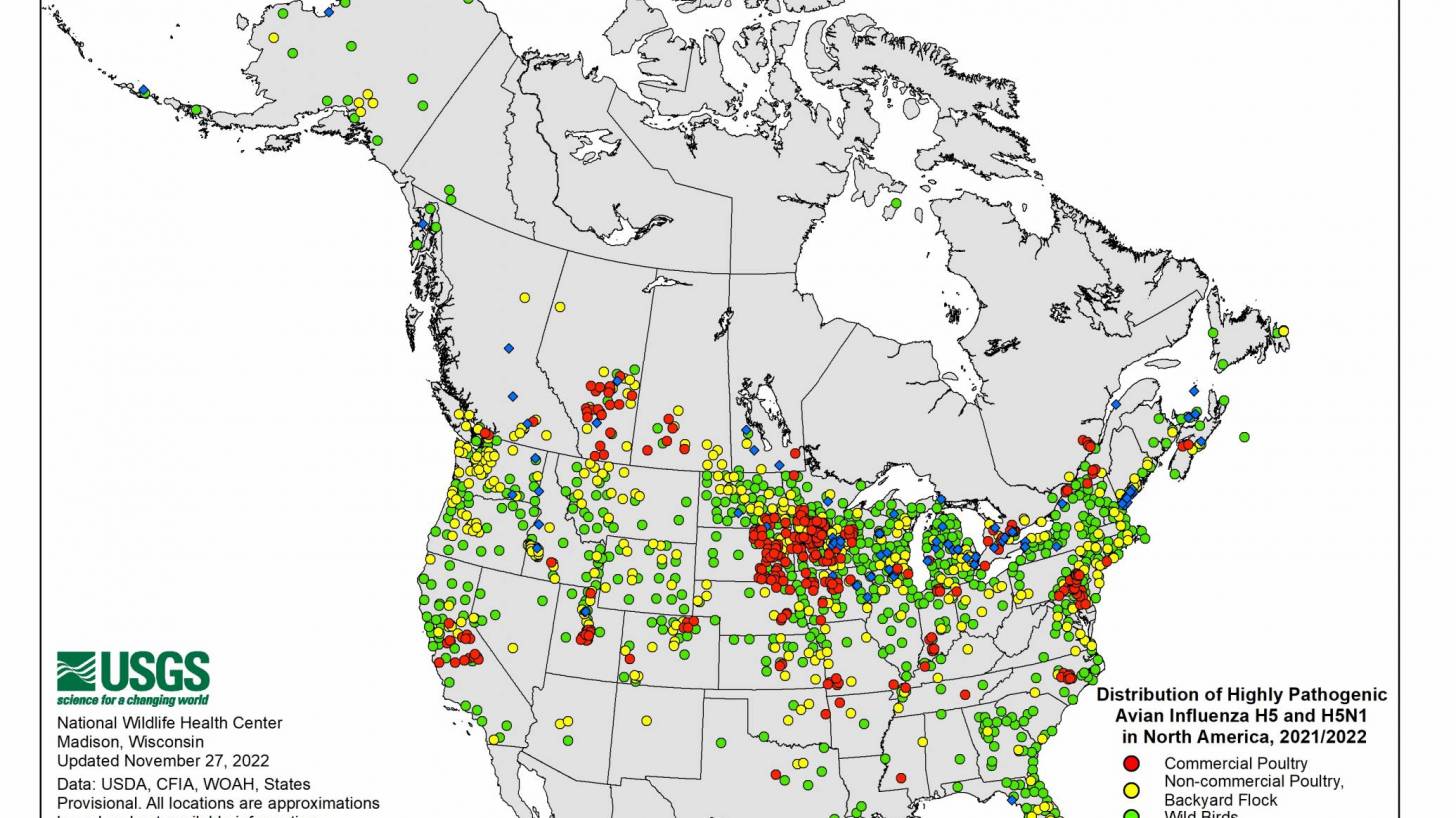
"But we must remain vigilant, especially as wild birds continue to pose disease risks. We all must recognize the important role biosecurity plays in limiting the impact of wild birds at farms and facilities."
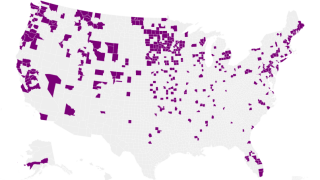
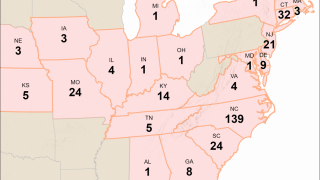
As of March 30, 2023, H5N1 viruses (clade 2.3.4.4b) have been detected in various birds in 16 countries in Latin America, the Caribbean, the United States, Canada, and multiple other countries.
Eleven human influenza A H5N1 2.3.4.4b infections were reported globally during 2022-2023.
The U.S. Centers for Disease Control and Prevention Technical Report issued on March 17, 2023, confirmed the current risk to the public from HPAI A(H5N1) viruses remains low.
For people, the U.S. Food and Drug Administration authorized CSL Seqirus' Audenz™ monovalent cell-based vaccine in January 2020.
And the U.S. government has awarded additional bird flu vaccine candidate studies.
Our Trust Standards: Medical Advisory Committee