UPDATE: Human Infection with Bird Flu in Chile

The U.S. Centers for Disease Control and Prevention (CDC) today published an addendum to Technical Report: Highly Pathogenic Avian Influenza A(H5N1) Viruses, which provides a summary of the case and the genomic analysis of the avian influenza virus from the first H5N1 infection reported in a human in Chile.
Recent tests completed on the Chilean man infected with bird flu indicate that this avian virus has partially adapted to spread between mammals, such as bears, cats, and dogs.
Similar to the original 'bird flu' report published on March 17, 2023, the CDC says despite the wide geographic spread of highly pathogenic avian influenza (HPAI) A(H5N1) viruses in wild birds and to poultry since 2022, with sporadic spillover to mammals, only a small number of sporadic human cases of A(H5N1) have been identified.
As of April 17, 2023, all reported human cases were associated with poultry exposures, and no cases of human-to-human transmission have been identified.
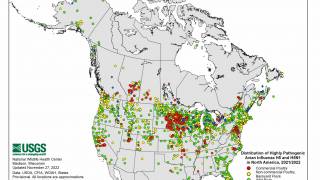
To date, HPAI A(H5N1) viruses are circulating in birds and poultry, with spillover to mammals.
However, those that have caused human infections cannot readily bind to receptors that predominate in a person's upper respiratory tract.
In the U.S., more than 6,300 people in 52 jurisdictions have been monitored since 2022, and only one human case has been identified in Colorado.
Therefore, the CDC says the current risk to the public from HPAI A(H5N1) viruses remains low.
However, continued sporadic human infections are anticipated because of the potential for influenza viruses to evolve rapidly and the vast global prevalence of HPAI A(H5N1) viruses in wild birds and poultry outbreaks.
Continued comprehensive surveillance of these viruses in wild birds, poultry, mammals, and people worldwide and frequent reassessments are critical to determining the public health risk and ongoing preparedness efforts.
From a vaccine perspective, the CDC has already taken action.
An H5 candidate vaccine virus (CVV) produced by the CDC is nearly identical or, in many samples, similar to the hemagglutinin (HA) protein of recently detected clade 2.3.4.4b HPAI A(H5N1) viruses in birds and mammals.
If needed, this CVV could produce a vaccine for people and provide reasonable protection against the clade 2.3.4.4b HPAI A(H5N1) viruses circulating in birds.
Furthermore, this H5 CVV is available and was shared with vaccine manufacturers.
Other bird flu vaccines, such as Audenz™, have already been U.S. FDA-approved.
These vaccines offer different protections than annual flu shots, which are not designed to prevent bird flu infections.
Note: The full summary of the human case in Chile is available at this link.
Our Trust Standards: Medical Advisory Committee