Children Protected From Typhoid With One Vaccine Dose
The World Health Organization (WHO) says an estimated 9 million people get sick from typhoid, and 110,000 people die from Salmonella Typhi, commonly known as typhoid.
In 2024, millions of children who lack access to safe drinking water and adequate sanitation are at risk in typhoid-endemic countries, particularly in Asia and sub-Saharan Africa.
Furthermore, travelers to these countries are usually at risk when exposed to low food hygiene standards and poor water quality, says the WHO.
The good news is that approved typhoid vaccines are available in 2024.
But, the duration of effectiveness has been debated for years.
A peer-reviewed study by researchers with the University of Maryland School of Medicine (UMSOM) helps clarify this debate.
Published in The Lancet on January 25, 2024, a phase 3 clinical trial found a single dose of the Vi polysaccharide conjugated to tetanus toxoid vaccine (Vi-TT) is durably efficacious for at least four years among children and shows efficacy in all age groups, including children younger than two years.
"The newly published study supports the long-lasting impacts of a single dose of TCV, even in the youngest children, and offers hope of preventing typhoid in the most vulnerable children," said Kathleen Neuzil, MD, MPH, CVD Director, the Myron M. Levine, MD, DTPH, Professor in Vaccinology at UMSOM and coauthor of the current study, in a press release.
This study found that between February 2018 and September 2018, 28,130 children were vaccinated. After a median follow-up of 4.3 years, 24 (39.7 cases per 100 000 person-years) children in the Vi-TT group and 110 (182,7 cases per 100 000 person-years) children in the MenA group were diagnosed with a first episode of blood culture-confirmed typhoid fever.
In the intention-to-treat population, the efficacy of Vi-TT was 78.3% (95% CI 66·3–86·1), and 163 (129–222) children needed to be vaccinated to prevent one case.
These results support current WHO recommendations in typhoid-endemic areas for mass campaigns among children aged nine months to 15 years, followed by routine introduction in the first two years of life.
Jeri Beales MSN, RN provides education and vaccinations to travelers preparing for their international adventures at Destination Health Travel Clinic near Boston, MA, and offered this advice about typhoid fever to Precision Vax on January 28, 2024, "Trying local cuisines and flavors is the highlight of many international adventures."
"But the food you eat is only as safe as the hygiene of the person preparing it."
"If you have plans to visit a country where typhoid fever is a problem, take the time to get the vaccine because a lot of the risk is literally out of your hands."
"The typhoid fever vaccine is not 100% effective and doesn't protect against all food-borne illness, so you should still be selective in what foods and beverages you consume."
A brief history of typhoid vaccines was discussed in a related Commentary written by Birkneh Tilahun Tadesse, Rita Soares, Barbosa Cardona, and Florian Marks.
Since the 19th century, the first generation vaccine was a whole cell inactivated version, which became less preferred due to significant adverse events.
Second-generation typhoid vaccines included live attenuated vaccines like Ty21a, which can be used in individuals older than six years, and capsular polysaccharide vaccines, which are less immunogenic in children younger than two years.
These limitations prompted the development of typhoid conjugate vaccines (TCVs), which have been shown to induce robust immune responses.
Currently, TCVs that have received WHO prequalification The Typbar TCV® vaccine, developed by Bharat Biotech, received WHO prequalification in 2017.
A University of Oxford study found that the vaccine is safe, 100% immunogenic, and prevents up to 87.1% of infections when using real-life definitions of typhoid fever.
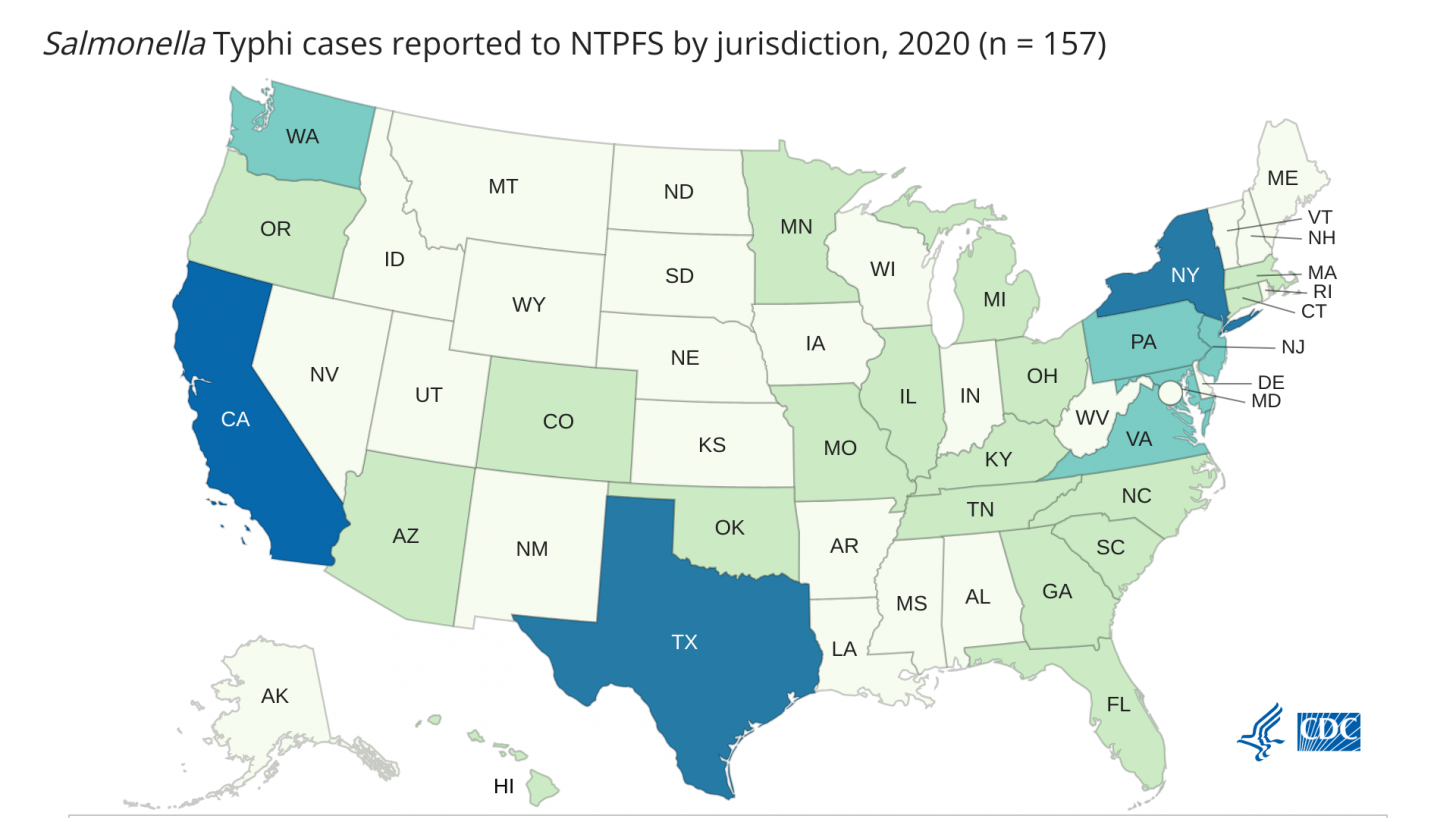
In the United States, very few typhoid cases are reported (157; 2020), mainly related to international travel.
However, the CDC says two typhoid vaccines are available in the U.S.
One is an inactivated (killed) vaccine; the other is a live, attenuated (weakened) vaccine.
Healthcare providers such as doctors, pharmacists, and nurses can help you decide which TCV is best for you.
Unfortunately, there isn't a vaccine that protects people against paratyphoid fever.
The Bill & Melinda Gates Foundation funds the Typhoid Vaccine Acceleration Consortium.
Our Trust Standards: Medical Advisory Committee