Typhoid Vaccine Now Available for Infants

The World Health Organization (WHO) announced the prequalification of the first conjugate vaccine for typhoid for children over 6 months of age in endemic countries.
Preceding this WHO prequalification, a study was published in The Lancet which was the first clinical trial to show that immunization with the typhoid conjugate vaccine (TCV) or Typbar-TCV is safe, well tolerated and will have a significant impact on disease incidence in endemic areas that introduce it.
The existing typhoid vaccines are not approved for children younger than 2 years of age.
Prequalified by WHO means that the Tybar-TCV vaccine met quality, safety and efficacy standards, and is eligible for procurement by various UN agencies.
Prequalification is a crucial next step to enable vaccines to become available in low-income countries, where the need is the greatest.
Typhoid conjugate vaccines (TCVs) are innovative products that have longer-lasting immunity than older vaccines, require fewer doses, and can be given to young children through routine childhood immunization programs, says the WHO.
The Strategic Advisory Group of Experts (SAGE) also called for the introduction of TCV to be prioritized for countries with the highest burden of typhoid disease or of antibiotic resistance to Salmonella Typhi, the bacterium that causes the disease.
Use of the vaccine should also help curb the frequent use of antibiotics for treatment of presumed typhoid fever, and thereby help to slow the alarming increase in antibiotic resistance in Salmonella Typhi.
Typhoid fever is a serious disease spread by contaminated food and water.
Typhoid symptoms of typhoid include lasting high fevers, weakness, stomach pains, headache, and loss of appetite. Some patients have constipation, and some have a rash. Internal bleeding and death can occur but are rare, reports the Centers for Disease Control and Prevention (CDC).
Typhoid fever is common in most parts of the developing world. Travelers to Asia, Africa, and Latin America are especially at risk, and the highest risk for typhoid is in South Asia.
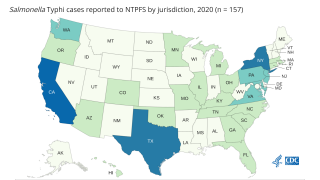
About 300 people get typhoid fever in the United States each year. Most typhoid cases in the USA are from people who have recently traveled internationally.
About 22 million cases of typhoid fever and 200,000 related deaths occur worldwide each year.
Typhoid vaccine is recommended for:
- Travelers to parts of the world where typhoid is common (Note: typhoid vaccine is not 100% effective and is not a substitute for being careful about what you eat or drink).
- People in close contact with a typhoid carrier.
- Laboratory workers who work with Salmonella Typhi bacteria.
Typhoid vaccine is available in pills or a shot and is only 50%-80% effective, says the CDC.
There are two vaccines to prevent typhoid. One is an inactivated (killed) vaccine gotten as a shot, and the other is a live, attenuated (weakened) vaccine, which is taken orally.
Inactivated Typhoid Vaccine (Shot)
- Should not be given to children younger than two years old.
- One dose provides protection. It should be given at least two weeks before travel to allow the vaccine time to work.
- A booster dose is needed every two years for people who remain at risk.
Live Typhoid Vaccine (Oral)
- Should not be given to children younger than six years old.
- Four doses, given two days apart, are needed for protection. The last dose should be given at least one week before travel to allow the vaccine time to work.
- A booster dose is needed every five years for people who remain at risk.
The CDC Vaccine Price List provides private sector vaccine prices for general information. Vaccine discount information can be found here.
Vaccines, like any medicine, can have side effects, says the CDC. You are encouraged to report negative side effects of vaccines to the FDA or CDC.
Our Trust Standards: Medical Advisory Committee