Just One Typhoid Vaccination Found Very Effective in Nepal

An extensive study of the typhoid conjugate vaccine (TCV) has shown a single dose to be 81 percent safe and effective in reducing typhoid in children between the ages of 9 months and less than 16 years.
Announced on December 5, 2019, and published in the New England Journal of Medicine, this interim analysis involved 20,019 children who were randomly given one of two vaccines; half received TCV and half received the Group A meningococcal (MenA) vaccine, which was the study’s control group.
Additionally, in this study’s immunogenicity subgroup, the seroconversion (a Vi IgG level that at least quadrupled 28 days after vaccination) was reported at 99 percent in the TCV group and 2 percent in the MenA vaccine group.
The researchers noted that these were preliminary results and that the study will continue to follow the participants for the next 2 years.
These results show the TCV vaccine has the potential to significantly reduce the burden of typhoid in high-risk populations. Typbar TCV is a vaccine containing polysaccharide of Salmonella typhi Ty2 conjugated to Tetanus Toxoid.
An estimated 26 million cases of typhoid fever and 5 million cases of paratyphoid fever occur worldwide each year, causing about 215,000 deaths.
Furthermore, this data is especially timely with the recent spread of extensively drug-resistant typhoid, which threatens child health in affected regions.
To address this need, the World Health Organization (WHO) issued a revised policy on typhoid vaccines in 2018. This policy follows evidence-based recommendations that prevention through vaccination is one of the most effective solutions to reduce the burden of typhoid in endemic areas.
The good news is the government of Pakistan said it was launching an Expanded Program on Immunization for the TCV vaccination campaign in the Sindh province.
Andrew Pollard, Professor of Paediatric Infection and Immunity at Oxford University’s Department of Paediatrics, said in a related statement, 'This is the first study to show that a single dose of TCV is safe, immunogenic, and effective, which provides clear evidence that vaccination will help efforts to control this serious disease and is a strong endorsement of the WHO policy for vaccine implementation.'
'The efficacy of these results in an endemic population adds to a growing body of evidence supporting the use of TCV to reduce disease and save lives in populations that lack clean water and improved sanitation,' said Dr. Kathleen Neuzil, M.D., director of the Center for Vaccine Development and Global Health at the University of Maryland School of Medicine and director of TyVAC.
The US Centers for Disease Control and Prevention (CDC) Advisory Committee on Immunization Practices recommends 2 typhoid fever vaccines, an oral vaccine, Vivotif, and an injectable vaccine, Typhim VI.
Neither vaccine is 100 percent effective, so travelers to endemic countries should also practice safe eating and drinking habits while traveling abroad, says the CDC
Caused by the bacterium Salmonella Typhi, Typhoid fever is a serious disease that is spread by contaminated food and water. Humans are the only source of these bacteria.
Symptoms of typhoid fever often include high fever, weakness, stomach pain, headache, cough, and loss of appetite. People may have diarrhea or constipation.
The incubation period of typhoid and paratyphoid infections is 6–30 days, says the CDC.
If you travel abroad and get sick, seek medical care ASAP.
If you get sick after returning to the United States, seek medical care and tell your health care provider where and when you traveled, says the CDC.
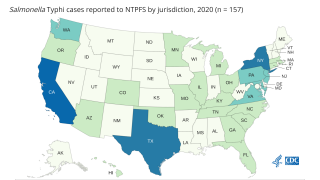
In the USA from 2008–2015, approximately 350 culture-confirmed cases of typhoid fever and 90 cases of paratyphoid fever caused by Paratyphi A were reported each year.
Funding for this study was provided by a grant from the Bill & Melinda Gates Foundation.
The investigational vaccine Typbar-TCV is licensed by Bharat Biotech International Limited, Hyderabad, India.
Travel vaccine news is published by Vax-Before-Travel
Our Trust Standards: Medical Advisory Committee