Malaria Vaccine Changed the Game, Awarded Innovation of the Year

Time USA LLC recently announced its list of the 'Best Inventions of 2024', identifying 200 innovations that changed people's lives.
On October 31, 2024, Time revealed it had selected an innovative vaccine that may save hundreds of thousands of lives each year.
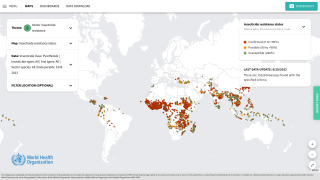
Earlier this year, the World Health Organization (WHO) recommended a second vaccine to combat malaria outbreaks, which sickens hundreds of millions and kills about 600,000 people each year.
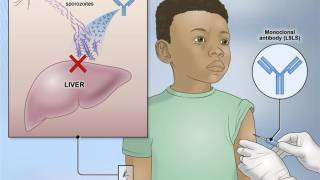
The Serum Institute of India Pvt. Ltd.'s R21/Matrix-M™ vaccine was released in 2023 following initial development by researchers at Oxford University. Serum began shipping the vaccine in May 2024 and anticipates distributing over 50 million doses annually.
R21 also includes the Matrix-M adjuvant from Maryland-based Novavax Inc., which amplifies the immune response generated by the vaccine.
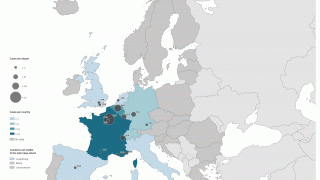
John Jacobs, Novavax's President, and CEO, said in a press release on July 15, 2024, "The introduction of the R21/Matrix-M™ malaria vaccine in Côte d'Ivoire marks a breakthrough in the fight to protect vulnerable children against a leading cause of death across the region while reinforcing our mission to create innovative vaccines that improve public health."
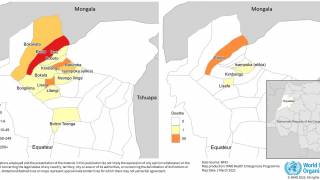
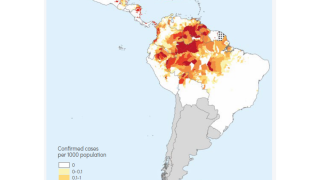
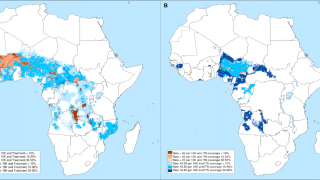
As of November 1, 2024, the WHO says the African Region shoulders the heaviest burden of this mosquito-transmitted disease this year in countries such as Burkina Faso, Cameroon, Ethiopia, the Democratic Republic of the Congo, Ghana, Mali, Mozambique, Niger, Nigeria, Sudan, Uganda, and Tanzania.
To inform travelers about global health risks such as malaria, the U.S. CDC publishes Travel Health Notices.
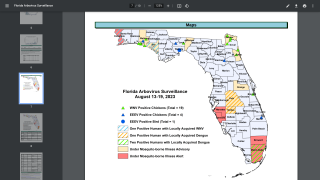
In the United States, the CDC has confirmed 1,576 malaria cases during 2024, mostly in international travelers arriving from Africa in New York City, Miami, Florida, and Los Angeles, California.
While the WHO recommends both malaria vaccines when visiting malaria-endemic areas, neither is available in the U.S.
Note: TIME Best Inventions has a paid application process through which companies may submit their products and organizations for editorial consideration. Applying does not guarantee inclusion. All decisions throughout the editorial process—from selecting candidates to finalizing the pieces on their achievements—are made by TIME's journalists based on relevancy and core editorial principles.
Our Trust Standards: Medical Advisory Committee