Preconception Malaria Immunization Found Safe

The U.S. National Institutes of Health (NIH) recently supported clinical trials of an experimental malaria vaccine in adults and found that all three tested regimens were safe.
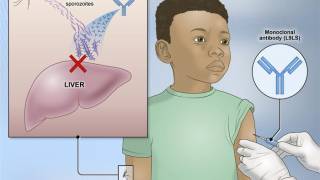
One of the trials enrolled healthy women who anticipated becoming pregnant soon after immunization.
That trial began with drug treatment to remove malaria parasites, followed by three injections spaced over a month of either saline placebo or the investigational vaccine at one of two dosages.
Both dosages of the vaccine candidate conferred a significant degree of protection from parasite infection, and clinical malaria was sustained for two years without the need for a booster dose, which was the first for any malaria vaccine.
In an exploratory analysis of women who conceived during the study, the vaccine significantly protected them from malaria during pregnancy. If confirmed through additional clinical trials, the approach modeled in this study could lead to improved ways to prevent malaria during pregnancy.
The researchers speculate that the PfSPZ Vaccine might avert malaria-related early pregnancy losses since parasitemia risk during the periconception period was reduced by 65 to 86%.
This is an important finding since malaria parasites, including those of the species Plasmodium falciparum (Pf), can cause illness in people of any age, including pregnant women and infants, who are especially vulnerable to life-threatening diseases.
Malarial parasitemia in pregnancy is estimated to cause up to 50,000 maternal deaths and 200,000 stillbirths in Africa each year.
Investigators from the NIH’s National Institute of Allergy and Infectious Diseases (NIAID) and the University of Sciences, Techniques and Technologies, Bamako, Mali, co-led the trials.
The vaccine candidate was the PfSPZ Vaccine, a radiation-attenuated vaccine based on Pf sporozoites (a stage of the parasite’s lifecycle) manufactured by Sanaria Inc.
Previous clinical trials of the PfSPZ Vaccine have shown it to be safe.
Results published in 2022, for example, an NIAID-sponsored, placebo-controlled trial of a three-dose regimen of PfSPZ Vaccine in Burkina Faso found that the vaccine had up to 46% efficacy that lasted at least 18 months.
“Preconception immunization is a new strategy to reduce mortality for women with malaria in pregnancy,” the researchers noted in a press release on August 14, 2024.
They plan to investigate the safety of the PfSPZ Vaccine administered during pregnancy, then examine the efficacy of PfSPZ given preconception or during pregnancy in more extensive clinical trials.
“Existing measures are not protecting women from malaria in pregnancy,” they added. “A safe and effective vaccine is urgently needed, and our results indicate PfSPZ Vaccine might be a suitable candidate,” they conclude.
As of August 2024, there are two WHO-recommended malaria vaccines. The Serum Institute of India has manufactured about 25 million R21/Matrix-M™ vaccines for distribution in Africa.
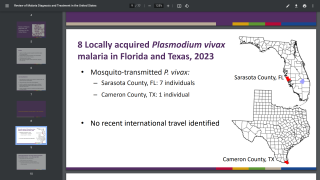
Malaria vaccines are offered in Africa but not in the United States.
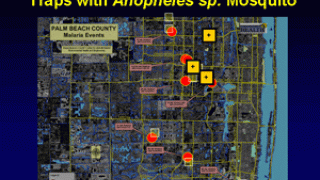
About 2,000 malaria cases are confirmed in the U.S. related to international travel. For example, Florida has reported 31 such cases, primarily from travelers to Africa as of August 2024.
Our Trust Standards: Medical Advisory Committee