Four Ebola Medications to be Clinically Tested in the DRC

The Ministry of Health of the Democratic Republic of the Congo (DRC) announced the launching of a randomized clinical trial to evaluate the effectiveness and safety of medications used in the treatment of Ebola patients.
This trial is the first-ever, multi-drug trial for the treatment of the Ebola Virus Disease, which is caused by an infection by a group of viruses within the genus Ebolavirus, such as:
- Ebola virus (species Zaire ebolavirus),
- Sudan virus (species Sudan ebolavirus),
- Taï Forest virus (species Taï Forest ebolavirus, formerly Côte d’Ivoire ebolavirus),
- Bundibugyo virus (species Bundibugyo ebolavirus).
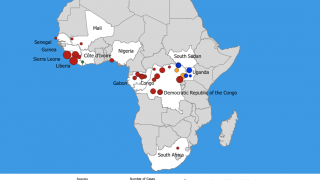
This is good news since the current Ebola outbreak in the DRC is close to becoming the 2nd largest outbreak recorded.
The current record is 425 Ebola cases during 2016, reports the US Centers for Disease Control and Prevention (CDC).
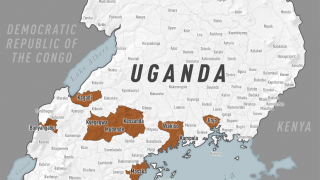
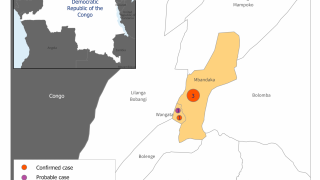
As of November 23, 2018, the DRC has reported 399 confirmed and probable Ebola cases since August 1, 2018.
Ebola is a rare and deadly disease spread by direct contact with blood or body fluids of a person infected with Ebola virus. It is also spread by contact with a contaminated object or infected animal, says the CDC.
This clinical study will form part of a multi-outbreak and multi-country study that was agreed to by partners under a World Health Organization (WHO) initiative.
“While our focus remains on bringing this outbreak to an end, the launch of the randomized control trial in DRC is an important step towards finding an Ebola treatment that will save lives,” said WHO Director-General Dr. Tedros Adhanom Ghebreyesus, in a press release.
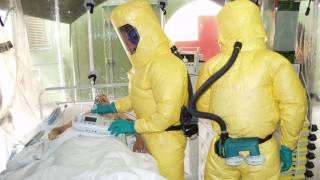
Until now, over 160 patients have been treated with investigational therapeutics under an ethical framework developed by WHO, in consultation with experts in the field and the DRC, called the Monitored Emergency Use of Unregistered and Investigational Interventions (MEURI).
The MEURI protocol was not designed to evaluate the efficacy of medications.
As of September 23, investigational agents had been administered to 38 patients — MAb114 (22), remdesivir (9), and ZMapp (7) reported an article in The New England Journal of Medicine.
Nineteen of these patients had been discharged, 12 had died, and 7 had remained hospitalized; those who died were in advanced stages of the disease when treatment was initiated.
Now that protocols for trials are in place, patients will be offered treatments under that framework in the facilities where the trial has started.
In others, compassionate use will continue up to the time when they join the randomization. Patients will not be treated noticeably differently from before, though the treatment they receive will be decided by random allocation.
“Our country is struck with Ebola outbreaks too often, which also means we have unique expertise in combatting it,” said Dr. Olly Ilunga, Minister of Health of the DRC.
“These trials will contribute to building that knowledge, while we continue to respond on every front to bring the current outbreak to an end.”
Moreover, the CDC upgraded its Travel Alert for the DRC to Level 2 status on October 19, 2018.
The current trial is coordinated by WHO, and led and sponsored by the DRC’s National Institute for Biomedical Research (INRB), in partnership with the DRC Ministry of Health, the National Institute of Allergy and Infectious Diseases (NIAID) which is part of the United States’ National Institutes of Health, The Alliance for International Medical Action (ALIMA) and other organizations.
Our Trust Standards: Medical Advisory Committee
- Democratic Republic of the Congo begins first-ever multi-drug Ebola trial
- WHO R&D Blueprint – Ad-hoc Expert Consultation on clinical trials for Ebola Therapeutics
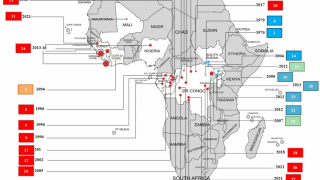
- Years of Ebola Virus Disease Outbreaks
- Ebola Zaire Vaccine Candidate Rolls Forward
- Ebola Outbreak in the DRC Records 139 Fatalities
- Ebola Vaccine Candidates Generating Positive Immune Responses