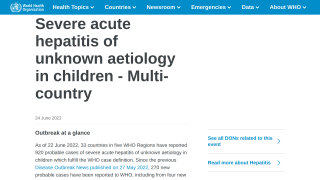
Liver Cancer Patients Can Benefit From Hep C Medications

A large clinical study conducted by researchers at UT Southwestern Medical Center (UTSWMED) refutes previous suggestions that Hepatitis C antiviral medications may lead to a higher recurrence of liver cancer.
This UTSWMED study, published in the journal Gastroenterology, found 42 percent of liver cancer survivors who were treated with direct-acting antivirals (DAAs) experienced a recurrence of their liver cancer, compared with 59 percent of patients who were not treated with antivirals.
Additionally, this UTSWMED study found no difference in the aggressiveness of the cancer in those patients who did experience a recurrence.
Dr. Amit Singal, Associate Professor of Internal Medicine and Medical Director of the Liver Tumor Program, said in a press release, “Based on these new data, providers can feel reassured that it is safe to treat hepatitis C in these patients and allow them to receive the known benefits of hepatitis C therapy.”
Effective antiviral medications have been available to treat hepatitis C infection in the USA since 2013.
This news is important since approximately 3.2 million individuals in the USA have chronic hepatitis C infection, which is also one of the leading causes of liver cancer.
And, many of these individuals struggle with inflammation of the liver and impaired liver function, as well as cirrhosis, or scarring of liver tissue.
The rate of new cases of liver cancer has been rising steadily in recent decades, and the state of Texas has one of the highest rates of occurrence in the country.
According to the Centers for Disease Control and Prevention (CDC), half of all individuals with liver cancer have underlying chronic hepatitis C infection.
When liver cancer is diagnosed early, it can be effectively treated with surgery, ablation, or radiation therapy.
Sometimes liver cancer patients have their tumor successfully removed, but the underlying chronic hepatitis C infection remains and continues to impair liver function further.
To lower your risk for liver cancer, get vaccinated against Hepatitis B, says the CDC.
The hepatitis B virus vaccination is usually given as a series of 3 shots over 6 months. The entire series of shots are needed for long-term protection.
Hepatitis B vaccines include ENGERIX-B, Recombivax HB, and HEPLISAV-B.
“Our results suggest that use of DAA therapies is safe and potentially beneficial in hepatitis C-infected patients with a history of liver cancer,” said Dr. Singal.
Funding for this study was provided by the National Cancer Institute and AbbVie Inc.
Dr. Singal has served on advisory boards and has received funding from Gilead and AbbVie. He has previously participated in a speakers’ bureau for Gilead. Other disclosures unrelated to this research appear in Gastroenterology.
UT Southwestern, one of the premier academic medical centers in the nation, integrates pioneering biomedical research with exceptional clinical care and education.
Our Trust Standards: Medical Advisory Committee