Hepatitis B Vaccine Delivered Positive Seroprotection Rate

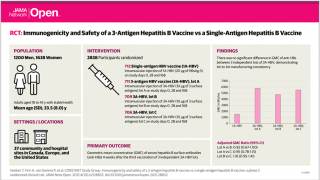
A California-based biopharmaceutical company announced immunogenicity and safety results of the ongoing clinical trial evaluating HEPLISAV-B®, a Hepatitis B Vaccine (HVB) in patients undergoing hemodialysis.
Dynavax Technologies Corporation published final immunogenicity data in 119 patients in this clinical trial evaluating a 4-dose regimen of HEPLISAV-B in adults with end-stage renal disease (ESRD) undergoing hemodialysis, demonstrated a seroprotection rate of 89.3 percent with high levels of anti-HBs antibodies, which are critical to maintaining protection in patients undergoing hemodialysis.
And, the interim safety data showed HEPLISAV-B was well tolerated, and no safety concerns were observed. Full safety data are expected by the end of 2021.
HEPLISAV-B is an adult hepatitis B vaccine that combines hepatitis B surface antigen with Dynavax's proprietary Toll-like Receptor (TLR) 9 agonist adjuvant CpG 1018 to enhance the immune response.
HEPLISAV-B is indicated to prevent infection caused by all known subtypes of hepatitis B virus in adults age 18 years and older.
Robert Janssen, M.D., Chief Medical Officer at Dynavax, stated in a press release, "We believe the 4-dose regimen of HEPLISAV-B, we are evaluating in this study, can provide an important hepatitis B vaccination alternative for patients undergoing hemodialysis by delivering high levels of protection with fewer doses compared to the current standard of care which requires up to 8 injections to complete the regimen."
The study, HBV-24, is designed to evaluate the immunogenicity of HEPLISAV-B at study week 20 and safety over the 68-week study duration. The safety and effectiveness of HEPLISAV-B have not been established in adults on hemodialysis.
Hepatitis B is a viral disease of the liver that can become chronic and lead to cirrhosis, liver cancer, and death. HBV is 50 to 100 times more infectious than HIV, and there is no cure for hepatitis B, says the U.S. CDC.
In adults, hepatitis B is spread through contact with infected blood and unprotected sex with an infected person. The CDC recommends vaccination for those at high risk for infection due to their jobs, lifestyle, living situations, and travel to certain areas.
Because people with diabetes are particularly vulnerable to infection, the CDC recommends vaccination for adults age 19 to 59 with diabetes as soon as possible after their diagnosis. And for people age 60 and older with diabetes at their physician's discretion.
The company says, ‘do not administer HEPLISAV-B to individuals with a history of a severe allergic reaction after a previous dose of any hepatitis B vaccine or to any component of HEPLISAV-B, including yeast.
HEPLISAV-B may not prevent hepatitis B infection in individuals who have an unrecognized hepatitis B infection at the time of vaccine administration.
Dynavax is a commercial-stage biopharmaceutical company developing and commercializing novel vaccines.
PrecisionVaccinations publishes research-based vaccine news.
Our Trust Standards: Medical Advisory Committee