FluMist Flu Vaccine Not Available, Yet

While millions of influenza vaccines have already been released to nurses, doctors, and pharmacists, one vaccine has yet to be released by the Food and Drug Administration (FDA).
As of August 30, 2018, the FluMist vaccine has not received FDA distribution approval.
*Interested in an Asthma clinical trial, click here*
The FluMist Quadrivalent live attenuated influenza vaccine (LAIV4) is an approved vaccination option for the 2018-19 flu season, according to the Centers for Disease Control and Prevention's (CDC) Advisory Committee on Immunization Practices (ACIP).
This ACIP approval is a change from the last 2 seasons.
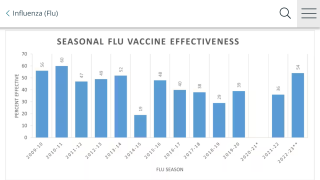
According to the ACIP, the previous concern was the FluMist vaccine effectiveness.
The ACIP reviewed data from 3 sources in February 2018.
Two observational data indicated that LAIV was poorly effective against influenza A(H1N1)pdm09-like viruses, and was significantly less effective than IIV against these viruses.
However, LAIV was effective against influenza B viruses, and effectiveness of LAIV and IIV against influenza A(H3N2) viruses generally did not differ significantly.
No estimates of the effectiveness of the current formulation of LAIV4, which contains a new H1N1pdm09-like vaccine virus, were available at the time of this review.
The 3rd source was manufacturer data concerning shedding and immunogenicity associated with the administration of LAIV containing the new H1N1pdm09-like vaccine virus, A/Slovenia/2903/2015, among children aged 24 months through less than 4 years of age.
These data suggest that this new H1N1pdm09-like virus has improved replicative fitness over previous H1N1pdm09-like viruses included in LAIV.
Separately, researchers reported that they found no evidence of increased risk of subsequent asthma diagnosis among children younger than 3 years of age who received a LAIV, administered as a nasal spray.
This vaccine’s shipping delay may negativity impact the Centers for Disease Control and Prevention (CDC) suggestion that everyone over 6 months of age receive the annual flu shot prior to November 1st.
The CDC says, to avoid missed vaccination opportunities, healthcare providers should offer flu shots to their patients during routine visits and hospitalizations.
This CDC recommendation follows a particularly severe 2017-18 season during which 179 children in the USA died from influenza-associated illness. Approximately 80 percent of these children were unvaccinated, reported the CDC.
When FluMist is released to pharmacies in the USA, vaccination appointments can be scheduled by visiting this page.
The CDC Vaccine Price List provides the private sector prices for general information.
Flu vaccine discounts can be found here.
Vaccines, like any medicine, can have side effects. You are encouraged to report negative side effects of vaccines to the FDA or CDC.
Our Trust Standards: Medical Advisory Committee