Maternal Flu Shots Protect Both Mom and Baby

Pregnant women remain the highest priority group for annual influenza vaccination, and a new study adds more evidence to that focus.
This new study in England found that when pregnant women receive an influenza vaccination, their future infants experience a reduced number of flu cases and related hospitalizations prior to reaching 6 months of age.
The Journal of Infectious Diseases published this study’s findings on November 9, 2019, based on infants in England, using data from the Clinical Practice Research Datalink pregnancy register, matched on week-of-birth and region and adjusted for ethnicity.
The new study’s results are as follows:
- The flu vaccine effectiveness (VE) was 66% during the 2013–2014 season and the VE against the dominant circulating influenza strain was higher at 78% against H1N1 in 2013–2014.
- The VE was 50% in 2014–2015, with similar VE against influenza-related hospitalization and 60% against H3N2 in 2014–2015.
Annual influenza immunization during pregnancy was first recommended by the US Surgeon General in 1960, mainly because the risk of influenza disease is high in pregnant women.
Influenza is more likely to cause severe illness in pregnant women, than in women of reproductive age who are not pregnant, said the US Centers for Disease Control and Prevention (CDC) on October 21, 2019.
Changes in the immune system, heart, and lungs during pregnancy make pregnant women, and women up to 2-weeks postpartum, more prone to severe illness from flu, including illness resulting in hospitalization.
The flu is also harmful to a pregnant woman’s developing baby.
A common flu symptom is a fever, which may be associated with neural tube defects and other adverse outcomes for a developing baby.
Also, getting vaccinated can help protect a baby after birth from flu, as the mother passes protective antibodies onto the developing baby during her pregnancy, says the CDC.
The CDC, the World Health Organization (WHO) and many national public health agencies recommend that pregnant women receive an influenza vaccination each year.
These organizations conduct studies to measure the benefits of seasonal flu vaccination each flu season to help determine how well flu vaccines are working. These VE studies regularly assess and confirm the value of flu vaccination as a public health intervention.
Study results of vaccine effectiveness can vary based on study design, outcome(s) measured, population studied and the season in which the flu vaccine was studied.
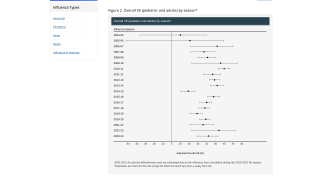
The overall, adjusted VE for influenza seasons from 2004-2018 is noted in this CDC chart.
Furthermore, influenza vaccine effectiveness for all vaccine types for the 2018-19 flu season can be found here.
If you get sick with flu symptoms call your doctor right away, says the CDC.
Early Treatment is Important for Pregnant Women:
- Treatment should begin as soon as possible because antiviral drugs work best when started early (within 48 hours after symptoms start).
- Antiviral drugs can make your flu illness milder and make you feel better faster. They may also prevent serious health problems that can result from flu illness.
- Oral oseltamivir is the preferred treatment for pregnant women because it has the most studies available to suggest that it is safe and beneficial.
- Antiviral drugs require a prescription from your doctor.
- Having a fever caused by flu infection or other infections early in pregnancy may be linked to birth defects in a baby.
- In addition to taking antiviral drugs, pregnant women who get a fever should treat their fever with Tylenol® (or store brand equivalent) and contact their doctor immediately.
And, there is no CDC recommendation for pregnant women to get written consent from their healthcare professional for influenza vaccination if they get vaccinated at a worksite clinic, pharmacy or other location outside of their physician’s office.
Furthermore, the CDC suggests pregnant women get a flu shot and not the nasal spray influenza vaccine for the current flu season.
The nasal spray flu vaccine is approved for use in non-pregnant individuals, 2 years through 49 years of age. But, people with some certain medical conditions should not receive the nasal spray flu vaccine.
Recent flu vaccine news
- Last Season's Influenza Virus Changed Personalities
- Influenza A Is Dominating in the Northern Hemisphere
As always, vaccination decisions should be part of an ongoing discussion between a provider and patient.
Influenza news published by Precision Vaccinations.
Our Trust Standards: Medical Advisory Committee
- Assessment of Effectiveness of Seasonal Influenza Vaccination During Pregnancy in Preventing Influenza Infection in Infants
- CDC: Pregnant Women & Influenza (Flu)
- Influenza Vaccine Effectiveness in Preventing Influenza-associated Hospitalizations During Pregnancy
- Year-round influenza immunisation during pregnancy in Nepal: a phase 4, randomised, placebo-controlled trial
- Brief education to increase uptake of influenza vaccine among pregnant women: a study protocol for a randomized controlled trial