Novel H3N2 Influenza DNA Vaccine Candidate Progresses in Mice Study

A vaccine manufacturer announced in a press release that its SynCon vaccine approach using a collection of DNA antigens generated broadly protective antibody responses against the most deadly strains of the H3N2 influenza viruses.
Additionally, this influenza vaccine candidate provided complete protection against heterologous lethal challenge, in a pre-clinical, mice study.
In a pursuit of overcoming the antigenic diversity of H3N2 viruses, Inovio Pharmaceuticals, Inc. developed a collection of H3HA DNA antigens and demonstrated broad, functional antibody responses against H3 viruses in mice.
Vaccination was also capable of inducing robust CD4 and CD8 T cell responses, which are reported to be critical for the prevention of disease in the elderly population.
Additionally, all (100%) vaccinated mice survived when infected with 10 times the lethal viral doses from two of the H3N2 virus which circulated during the 1968 and 1982 outbreaks, highlighting the strong protection afforded by Inovio’s H3HA vaccine.
Inovio is currently in discussions with third-party funders to support further development of the company’s technology with its advantages in promoting global human health.
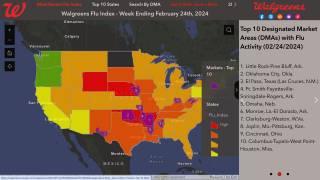
According to the Centers for Disease Control and Prevention (CDC), H3N2 hits people harder than other seasonal flu strains and can be especially deadly among vulnerable groups like the elderly and children.
Researchers still aren’t sure why, but they’ve found that a flu season involving the H3 virus is generally more virulent — with more hospitalizations and flu-related deaths — than seasons involving mostly H1N1 or influenza B viruses, says the CDC.
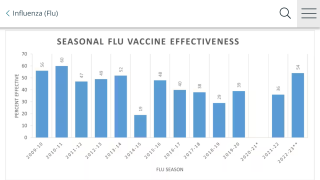
Furthermore, the H3 part of the current commercially available vaccine doesn’t just work poorly in older adults.
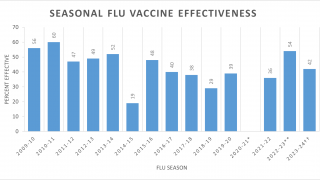
During the 2017/18 flu season, adults aged 18 to 49 got very little protection—13 percent—from the H3 component, reported the CDC.
The issue Inovio is hoping to resolve with this vaccine candidate is throughout the 2017-18 flu season the commercially available H3N2 vaccine efficacy was reported low due to the mismatch between the vaccine and circulating H3N2 viruses.
“In some populations, the vaccine showed only 13% effectiveness, which contributed to a much greater rate of pneumonia and flu-related deaths,” said Dr. Laurent Humeau, Inovio’s Senior Vice President, Research & Development.
“This study is a step towards conquering the diversity of the H3N2 flu viruses that has vexed researchers for years. In this preclinical study, Inovio demonstrated that its SynCon® method of antigen design is capable of providing protection against multiple H3N2 strains.”
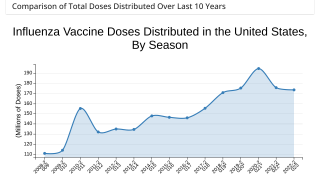
Earlier this year Inovio reported that its synthetic vaccine approach using a collection of synthetic DNA antigens generated broad protective antibody responses against all major deadly strains of H1 influenza viruses from the last 100 years including the virus that caused “Spanish Flu” in 1918 in multiple animal models including mice, guinea pigs, and non-human primates.
Regardless of previous flu vaccine effectiveness, the CDC’s Advisory Committee on Immunization Practices (ACIP) says the flu shot should be administered no later than the end of October.
Which means, most people can easily adhere to this CDC suggestion during their next visit to a pharmacy.
To find flu shot pricing information, the CDC Vaccine List provides the private sector prices for general information.
Flu vaccine discounts can be found here.
Vaccines, like any medicine, can have side effects, says the CDC. You are encouraged to report negative side effects of vaccines to the FDA or CDC.
Inovio Pharmaceuticals, Inc. is a late-stage biotechnology company focused on the discovery, development, and commercialization of DNA immunotherapies that transform the treatment of cancer and infectious diseases.
Our Trust Standards: Medical Advisory Committee