Flu Shot ‘Primer’ Study Launches in St. Louis

Researchers are testing an influenza vaccine “primer” that would be given about 3 months before the start of seasonal flu.
Seasonal flu shots are usually administered during October each year.
Because the influenza virus changes from year to year, the vaccine is often reformulated in anticipation of the changes.
This dynamic vaccine production process is one reason vaccine effectiveness can vary, depending on how well scientists predict which influenza strains will circulate and match the current flu shots.
The St. Louis University believe a vaccine primer called M2SR, may help overcome the production guessing the game.
“Giving the M2SR vaccine as a priming vaccination before a currently licensed vaccine may broaden the immune response,” said Dr. Daniel Hoft, director of the university's division of infectious diseases, allergy, and immunology.
The experimental vaccine also has the potential to be a safer and more protective vaccine than the 2 types of licensed seasonal flu vaccines, Dr. Hoft said in a St. Louis Post Dispatch article.
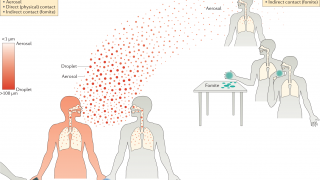
“The reasons to focus on pediatric populations for this M2SR vaccine are both to better protect children and to minimize viral transmission since children are a major cause for amplifying transmission."
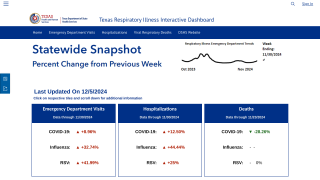
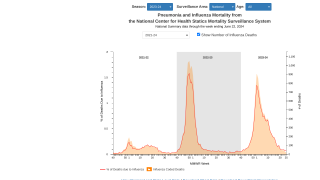
This is an important study since 116 flu-related, pediatric deaths have been reported during 2019.
To learn more about the vaccine study or enroll, call 314-977-6333 or email vaccine@slu.edu.
Recent flu vaccine news:
- FluMist Flu Vaccine Found Safe in Children With Asthma
- deltaFLU Vaccine Found Efficacious Against H5N1 Influenza
- Schedule a Flu Shot Before Visiting the Southern Hemisphere
Previously, during September 2018, a nasal influenza vaccine clinical trial was launched by the National Institutes of Health.
This is a Phase I double-blind study in 50 healthy adolescents designed to assess the safety and immunogenicity of a prime-boost regimen of H3N2 M2SR intranasal influenza vaccine, followed by the inactivated Quadrivalent Influenza Vaccine (QIV) booster.
The H3N2 M2SR is made from a strain of seasonal influenza virus (H3N2) that has been genetically designed to replicate only once in the body.
The H3N2 M2SR intranasal influenza vaccine candidate is manufactured by FluGen, Inc.
The CDC Vaccine Price List provides private sector prices for general information. Flu vaccine discounts can be found here.
Flu vaccines, like any medicine, can have side effects. You are encouraged to report negative side effects of vaccines to the FDA or CDC.
Our Trust Standards: Medical Advisory Committee